NEUROSURGICAL EMERGENCIES
JULIE K. MCMANEMY, MD AND ANDREW JEA, MD
GOALS OF EMERGENCY THERAPY
A patient with a neurologic disorder is considered a neurosurgical emergency when any delay of treatment may lead to serious permanent neurologic morbidity or death. Diagnostic tests and radiographic evaluation should be tailored to early recognition of neurosurgical emergencies to assist with clinical management and appropriate neurosurgical consultation.
KEY POINTS
 Intracerebral hemorrhage may accompany cerebral vascular malformations.
Intracerebral hemorrhage may accompany cerebral vascular malformations.
 Headache is a common presenting complaint.
Headache is a common presenting complaint.
 Pediatric hydrocephalus often requires surgical intervention.
Pediatric hydrocephalus often requires surgical intervention.
RELATED CHAPTERS
SPONTANEOUS (NONTRAUMATIC) INTRACEREBRAL HEMORRHAGE
CLINICAL PEARLS AND PITFALLS
• Approximately 5% of the population have cerebrovascular malformations including arteriovenous malformations, cavernous malformations, venous angiomas, and capillary telangiectasias.
• Unruptured aneurysms and certain types of cerebral vascular malformations are asymptomatic.
• Clinical presentations in infants may be nonspecific.
• Arteriovenous malformations (AVM) typically present with spontaneous hemorrhage and/or seizure.
Clinical Considerations
The presentation of the various types of cerebral vascular malformations may be insidious. Often, these malformations are asymptomatic. The presentation of infants may be very nonspecific and include poor feeding, vomiting, irritability, bulging anterior fontanelle, and altered mental status. Typical complaints in children include headache that may be localized, early morning awakening due to headache, progression of headache with increasing severity and/or frequency, vomiting, visual changes, neck stiffness, focal neurologic findings, altered mental status, seizure, lethargy, or obtundation. Signs of impending cerebral herniation include altered mental status, pupillary changes, bradycardia, hypertension, and respiratory depression.
Aneurysm
Current Evidence. Spontaneous dissecting aneurysms comprise about 45% of all aneurysms in children <15 years of age. The most typical presentation is with ischemia; however, they may also present with subarachnoid hemorrhage (SAH) or intracerebral hematomas. Spontaneous dissection of extra- or intracranial arteries has been associated with conditions such as Marfan syndrome, fibromuscular dysplasia, artherosclerosis, and moyamoya disease. The pathophysiology of spontaneous dissecting aneurysms seems to be multifactorial, including congenital, acquired, and hemodynamic factors.
Diagnostic Imaging. Because computed tomography (CT) is noninvasive and widely available, CT angiography (CTA) has been used for the screening and diagnosis of vascular injuries (Figure 130.1). The main disadvantage of CTA is related to bony artifact limiting its ability to identify injuries in some areas such as carotid canal or transverse foramina. However, current generation 16-detector scanners are capable of rendering very high–resolution images along with high-speed data acquisition.
Magnetic resonance imaging (MRI) and angiography (MRA) offers a high-resolution noninvasive approach for diagnosis and follow-up of vascular injuries. It is helpful in visualization of the arterial wall and detection of intramural hematoma. However, the accuracy of MRA is limited in detecting small intimal injuries (<25% luminal stenosis) and early pseudoaneurysm formation. The resolution of MRA now approaches that of conventional angiography.
Cerebral angiography remains the gold standard diagnostic modality. It is currently the most accurate modality as it provides fine detail of vascular anatomy and intimal injury near bony structures such as the skull base or the transverse foramen. However, due to its invasive nature and associated risk of iatrogenic injuries, it is advisable to reserve formal angiography for confirmation of findings detected on a screening diagnostic examination.
Management. Spontaneous dissecting aneurysms presenting with bleeding are uncommon, but should be treated because of a poor natural history. Aggressive surgical management with clipping, resection, or trapping of intracranial dissecting aneurysms by surgical or endovascular methods seems the most appropriate treatment.
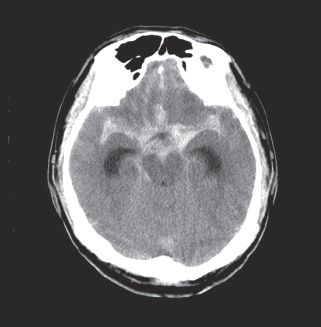
FIGURE 130.1 Axial CT of the brain shows hyperdensity consistent with aneurysm associated with acute hemorrhage in the basal cisterns.
Cavernous Malformation
Current Evidence. Cavernous malformations (CMs), also known as cavernous angiomas or cavernomas, are compact lesions comprised of sinusoidal vascular channels lined by a single layer of endothelium that lacks the full complement of mature vessel wall components. Between the vascular channels in the core of the lesion, there is loose connective tissue stroma without intervening brain parenchyma. The prevalence of CMs has been estimated to be between 0.4% and 0.9% of the population and 8% and 15% of all vascular malformations.
The majority of CMs are located supratentorially. Of the supratentorial CMs, most are located in the white matter of the cerebral hemispheres. The infratentorial CMs are located in the cerebellum, pons, midbrain, and medulla. Less frequent locations of CMs are the lateral and third ventricles, cranial nerves, and optic chiasm. Acute hemorrhage from a chiasmal CM is a rare cause of permanent visual loss. Of the extracerebral locations, the cavernous sinus, the orbits, and the spinal cord are the most common.
Diagnostic Imaging. CT is more sensitive at detecting CMs, but its specificity is low since most appear simply as high-density lesions with little or no contrast enhancement. This is in contrast to the high sensitivity and specificity of MRI for CMs. The MRI appearance of CMs has been categorized into four types: a hyperintense core on T1- and T2-weighted images representing subacute hemorrhage (Type I); a “classic” picture of mixed-signal, reticulated core surrounded by a low-signal rim (Type II); a iso- or hypointense lesion on T1 and markedly hypointense lesion with hypointense rim on T2, which corresponds to chronic hemorrhage (Type III); and punctate, poorly visualized hypointense foci, which can be visualized only on gradient echo MRI, representing tiny CM or telangiectasia (Type IV).
Management. With most asymptomatic CMs, particularly when the diagnosis is relatively clear by MRI characteristics, the right approach for the patient is conservative management with close follow-up. In contrast to a bleeding episode from an AVM, a bleeding episode from a CM is rarely life-threatening. However, there is more controversy with symptomatic cavernous malformations which hemorrhage in deep, difficult-to-access surgical locations.
AVM. Current Evidence. AVMs are vascular abnormalities leading to a fistulous connection of arteries and veins without a normal intervening capillary bed. In the cerebral hemispheres, they frequently occur as cone-shaped lesions with the apex of the cone reaching toward the ventricles. Nearly all AVMs are thought to be congenital. Supratentorial location is the most common (90%). The most common presentation of an AVM is intracerebral hemorrhage (ICH). After ICH, seizure is the second most common presentation. Other presentations of AVMs include headache and focal neurologic deficits which may be related to steal phenomena or other alterations in perfusion in the tissue adjacent to the AVM.
Size of AVM. In a series of 168 patients followed after presentation without a prior hemorrhage, the size of the AVM was not found to be predictive of future hemorrhage. However, other studies have found AVMs of small size to be at higher risk of hemorrhage.
AVMs and Aneurysms. Prevalence of the association of AVMs with aneurysms varies from 2.7% to 22.7%. This association seems to be correlated with a higher risk of hemorrhage. Brown et al. studied 91 patients with unruptured AVMs and found the risk of ICH in patients with coexisting aneurysm to be 7% at 1 year compared with 3% among those with AVM alone. At 5 years, the risk persisted at 7% per year, while it decreased to 1.7% per year in those with an AVM not associated with aneurysms.
Diagnostic Imaging. A CT scan may be used as an initial screening tool for patients presenting with neurologic sequelae related to unruptured or ruptured AVMs. This study can be used quickly to determine location of the lesion, acute hemorrhage, hydrocephalus, or areas of encephalomalacia from previous surgery or rupture. A nonenhanced CT may show irregular hyperdense areas frequently associated with calcifications in unruptured AVMs or acute hemorrhage with ruptured AVMs (Figure 130.2). A contrast enhanced CT can demonstrate the nidus, feeding vessels, or dilated draining veins.
MRI is superior to CT scan in delineating details of the macro architecture of the AVM, except in the case of acute hemorrhage. These architectural features include exact anatomic relationships of the nidus, feeding arteries, and draining veins as well as topographic relationships between AVM and adjacent brain. MRI is sensitive in revealing subacute hemorrhage. The AVM appears as a sponge-like structure with patchy signal loss, or flow voids, associated with feeding arteries or draining veins on T1-weighted sequences (Figure 130.3). MRI and angiography in combination provide complementary information that facilitates understanding the three-dimensional structure of the nidus, feeding arteries, and draining veins. MR angiography (MRA) currently cannot replace conventional cerebral angiography. In the case of acute hemorrhage, the hematoma obscures all details of the AVM making MRA virtually useless. This calls for direct use of cerebral angiography if the characteristics of the hematoma strongly suggest AVM as an etiology.
Management. The currently used treatments for AVMs include: (1) Microsurgical resection only, (2) preoperative endovascular embolization followed by microsurgical resection, (3) stereotactic radiosurgery only, (4) preprocedural endovascular embolization followed by radiosurgical treatment, (5) endovascular embolization only, and (6) observation only. The ultimate goal for all of these modalities is cure for the patient; however, the only way to achieve cure is with complete obliteration of the AVM. Microsurgery is the gold standard for resection of small superficial AVMs that other methods of treatment must be measured against. There is certainly a well-established role for adjunctive endovascular embolization of some AVM’s. Clearly, there are specific situations, such as small deep AVMs in eloquent brain structures, where microsurgery should not be used as the primary treatment modality; stereotactic radiosurgery and occasionally embolization (when there is reasonable expectation of complete obliteration by embolization) are the preferred treatment options in these cases. We also make a case for observation in patients with large AVM’s in or near critical areas of the brain that are not ideal for surgical resection or radiosurgery. Here, the pursuit of treatment may actually be more harmful to the patient than the natural history of the AVM.
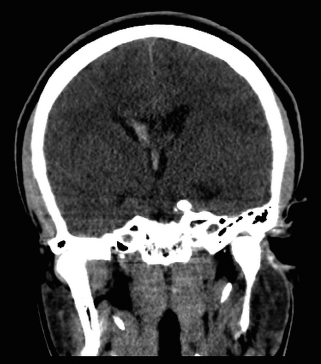
FIGURE 130.2 Coronal CT of the brain demonstrates intraventricular hemorrhage with communicating hydrocephalus with minimal interval increase in the ventricular dilatation. Diffuse cerebral edema with narrowing of the CSF space is suggestive of increased intracranial pressure.
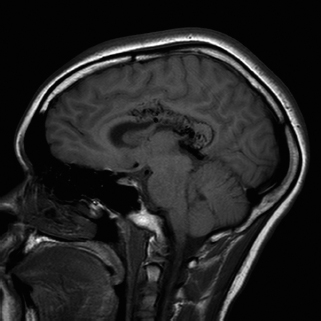
FIGURE 130.3 T1-weighted sagittal MRI demonstrates an AVM in the right corpus callosum with intraventricular hemorrhage with main feeding vessel from right pericallosal artery and draining into the right internal cerebral vein.
Indications for Surgical Resection. There are several clear indications for microsurgical resection of AVMs. AVMs with Spetzler–Martin grades I to III on the convexity should generally be resected. The Spetzler–Martin grading system takes into account three factors that greatly affect the surgical resectability of the AVM: size (<3 cm, 1 point; 3 to 6 cm, 2 points; >6 cm, 3 points), location (noneloquent cortex, 0 points; eloquent cortex, 1 point), and venous drainage (superficial only, 0 points; deep, 1 point). Patients with AVMs that present with major hemorrhage, progressive neurologic deterioration, inadequately controlled seizures, intractable headache, or venous restrictive disease should be strongly considered for surgical resection.
Cerebellar and pial brainstem AVMs should also be given strong consideration for surgical resection to prevent the higher risk of bleeding as compared to supratentorial AVMs. Some basal ganglia and thalamic AVMs should be surgically resected, as they carry a considerably higher annual bleed rate of 11.4%; in addition, morbidity and mortality with each bleed in these locations reach 7.1% and 42.9%, respectively (again, in contrast to the overall mortality rate of AVM hemorrhage of 10%).
Hence, one may justify a more aggressive approach for surgical treatment in younger patients as their cumulative risk of hemorrhage is so high. In addition, neurologic deficit caused at a young age is generally better tolerated and has a greater chance of recovery.
ACUTE HYDROCEPHALUS
CLINICAL PEARLS AND PITFALLS
• Hydrocephalus has been categorized as obstructive (noncommunicating) or nonobstructive (communicating).
• Most cases of pediatric hydrocephalus, even congenital, have a delayed diagnosis.
• Most children with hydrocephalus will need surgical treatment.
Hydrocephalus is the excess accumulation of cerebrospinal fluid (CSF), usually as the result of obstruction in CSF absorption, resulting in raised intracranial pressure (ICP). Cerebrospinal fluid is produced by the choroid plexus which is located within all four ventricles in the brain. Under normal conditions, the CSF exits the fourth ventricle to circulate in the subarachnoid space to be absorbed back into the venous system largely through arachnoid villi located at the superior sagittal sinus. Obstructive (or noncommunicating) hydrocephalus does not allow for the CSF to leave the ventricular system, and nonobstructive (or communicating) hydrocephalus occurs when the obstruction to CSF absorption lies outside the ventricular system in the subarachnoid space or at the arachnoid villi.
Common causes of obstructive hydrocephalus include stenosis of the cerebral aqueduct (from congenital causes, midbrain tumors, following hemorrhage or infection) and posterior fossa tumors. Common causes of nonobstructive hydrocephalus include scarring of the subarachnoid space and arachnoid villi following intraventricular hemorrhage (IVH) in premature infants or meningitis. In congenital conditions such myelomeningocele, the cause of hydrocephalus is likely multifactorial and may involve both obstructive and nonobstructive elements.
Current Evidence. Infections are a common cause of hydrocephalus in infants and children. An estimated 1% of pediatric patients who survive bacterial meningitis, including gram-negative organisms (particularly Escherichia coli) which occur most frequently in the neonatal age group, Haemophilus influenzae, Streptococcus pneumoniae, and group B streptococci, develop progressive hydrocephalus. Other less common infectious causes of hydrocephalus in children include tuberculosis meningitis whose worldwide prevalence is rising, toxoplasmosis (or other members of the TORCH group) usually diagnosed in the perinatal period, and viral meningitis and encephalitis. Head trauma has been recognized as a common cause of hydrocephalus. About 4% of patients develop posttraumatic hydrocephalus requiring surgical CSF diversion. True congenital hydrocephalus, meaning hydrocephalus present at birth, has an estimated incidence of 0.2 to 0.8/1,000 live births in the United States. The incidence of congenital hydrocephalus associated with conditions, such as Dandy–Walker malformation (approximately 85% to 95%), myelomeningocele (approximately 80% to 90%), and IVH of prematurity (approximately 35%), is better established.
Midline arachnoid cysts and tumors related to the ventricular system can cause hydrocephalus by obstruction of the CSF pathways. Tumors may also cause hydrocephalus by spilling blood or protein into the CSF, making the CSF more viscous, overloading the absorptive capacity of the arachnoid villi, and resulting in a communicating hydrocephalus.
Clinical Considerations
Clinical Recognition. Infants presenting symptoms include macrocephaly, bulging fontanelle, excessive irritability, lethargy, or vomiting. Sunsetting of the eyes may be present. This usually occurs later in the clinical course and consists of a spectrum of findings, including components of Parinaud syndrome (downward eye deviation, lid retraction, and convergence-retraction nystagmus). As raised ICP progresses, infants may develop bradycardia and/or apneic episodes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







