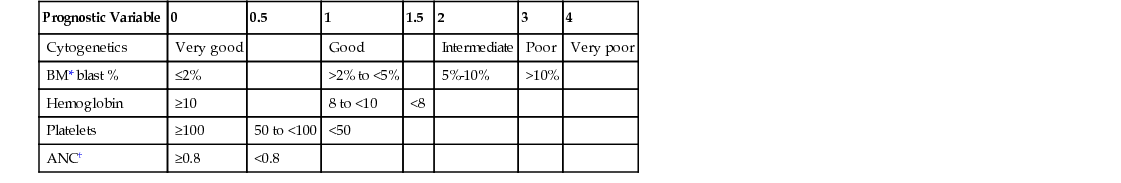
Anna D. Schaal The myelodysplastic syndromes (MDSs) are a heterogeneous group of bone marrow disorders characterized by ineffective dysplastic growth of the hematopoietic precursors. MDS is not one disease but a diverse series of hematologic conditions that have variable clinical presentation, biologic activity, and prognosis. There are three dominant features of this group of diseases. The first is impaired maturation of hematopoietic stem cells and increased cell death, or apoptosis. This is characterized by cytopenias. There also is a clonal expansion of the abnormal cell line, which manifests itself as progressive disease over time. Patients with MDS also have a variable risk of transformation to acute leukemia. MDS is a relatively rare disease. It is primarily a disease of older adults, with the annual incidence rate rising to 4.6 per 100,000 persons per year in those 60 years old and older. Approximately 15,000 to 45,000 new cases of MDS are diagnosed each year in the United States. Males are affected about 1.5 to 2 times as often as females.1 The knowledge of pathophysiologic abnormalities that contribute to MDS is advancing at a remarkable pace. Defects in cellular differentiation, responsiveness to cytokines and growth factors, altered hematopoietic microenvironment, increased apoptosis, and abnormal DNA methylation are all pathways that lead to the ineffective hematopoiesis that is the trademark of MDS. This ineffective hematopoiesis results in peripheral cytopenias despite a packed, hypercellular bone marrow. In essence, the bone marrow produces cells that are not able to mature into functional red blood cells, white blood cells, and platelets. The diagnostic classification of MDS has been a controversial topic for years. The World Health Organization has proposed a classification system. The acceptable categories include refractory anemia, refractory anemia with ringed sideroblasts, refractory cytopenia with multilineage dysplasia, 5q− syndrome, and myelodysplasia unclassifiable.2 Each category of MDS behaves in a different manner and has different treatment implications. The International Prognostic Scoring System–Revised (IPSS-R) is another useful classification system that is used to score the different variants of MDS (Table 241-1). The scoring is based on five factors: the percentage of blasts in the bone marrow, the specific karyotype or genetic abnormality risk group, hemoglobin, platelet count, and absolute neutrophil count (ANC). Corresponding scores that range from 0 to 6 help predict both survival and the risk of evolution to acute myeloid leukemia.2 The IPSS-R is used to help with projecting prognosis and making treatment decisions.3 TABLE 241-1 The IPSS-R Parameters and Their Score Values In general, the clinical presentation of MDS is related to symptoms of bone marrow failure or of the specific cytopenias that each patient is experiencing. If the red blood cells are the prominent lineage that is not maturing, then symptoms of anemia will be present. These may include fatigue, pallor, headaches, shortness of breath (or dyspnea) on exertion, and chest pains or palpitations. If the platelet count is low, easy bruising or bleeding will be the dominant presenting symptom. Frequent nose bleeds, petechiae, hematochezia, heavy menstrual bleeding, or large hematomas may encourage a patient to seek medical attention. Finally, if the white blood cells are not maturing, the main presenting symptoms will be of neutropenia, namely, frequent or severe infections. Splenomegaly is uncommon in MDS. Increasingly, more and more patients are being diagnosed incidentally from an abnormal complete blood count (CBC) done routinely in the primary care setting. In this case, patients feel well and have no subjective or physical examination findings that would make one suspect a myelodysplastic disorder. A number of specific diagnostic tests are required for the diagnosis of MDS. These include peripheral blood smear, bone marrow biopsy and aspirate with cytogenetic analysis, flow cytometry, and immunohistochemical staining. The peripheral blood smear will often reveal erythrocytes with anisocytosis, poikilocytosis, or basophilic stippling. The granulocytes may be larger than normal and may lack normal granulation. Platelets may also be larger than normal. The bone marrow biopsy almost always shows a hypercellular, packed marrow, which is in sharp contrast to the peripheral cytopenia. The hypercellular marrow often demonstrates defects in cellular maturation in all cell lines. For erythroid precursors, this includes megaloblastic cells, which are predominantly younger forms, and mature cells with distorted nuclei. Ringed sideroblasts will often be identified. These red blood cells are characterized by excessive cytoplasmic iron granules in a perinuclear distribution.4 Megakaryocytes are the polynuclear cells responsible for platelet production. In MDS, the megakaryocytes are often smaller than normal and hypolobulated. The precursors for white blood cells may also have abnormal granulation and deformed nuclei. Cytogenetic abnormalities play a role in prognosis. Cytogenetic abnormalities have been found in 50% to 60% of patients with de novo MDS and up to 80% of patients with therapy-related MDS.5 Common abnormalities include 5q− syndrome, which is a good prognostic indicator, and monosomy 7, which is associated with a poor prognosis and an increased risk of transformation to acute leukemia. Several disorders may mimic the morphologic changes of MDS and should be considered in the differential diagnosis. If anemia is the only presenting sign, common types of anemias must first be excluded, including folate and vitamin B12 deficiency, iron deficiency, anemia of chronic disease, renal failure, alcohol abuse, hereditary spherocytosis, and hemolysis. Infection, such as with human immunodeficiency virus (HIV), parvovirus, and Epstein-Barr virus, should also be ruled out. Drug toxicity or adverse reactions often imitate the cytopenias of MDS. Primary acute or chronic leukemia or a metastatic solid tumor with bone marrow metastasis should also be considered in the differential diagnosis. Other hematologic abnormalities often mimic MDS presentation, including aplastic anemia, Fanconi anemia, acute myeloid leukemia, myeloproliferative neoplasms and paroxysmal nocturnal hematuria. The bone marrow biopsy is the gold standard of diagnosis, because it often can exclude these disorders.
Myelodysplastic Syndromes
Definition and Epidemiology
![]() Specialist referral is indicated for all suspected cases of MDS.
Specialist referral is indicated for all suspected cases of MDS.
Pathophysiology
Prognostic Variable
0
0.5
1
1.5
2
3
4
Cytogenetics
Very good
Good
Intermediate
Poor
Very poor
BM* blast %
≤2%
>2% to <5%
5%-10%
>10%
Hemoglobin
≥10
8 to <10
<8
Platelets
≥100
50 to <100
<50
ANC†
≥0.8
<0.8
Risk Group
Risk Score
Very low
≤1.5
Low
>1.5-3
Intermediate
>3-4.5
High
>4.5-6
Very high
>6

Clinical Presentation and Physical Examination
Diagnostics
Differential Diagnosis
Myelodysplastic Syndromes
Chapter 241







