Fig. 15.1
Oblique view of transforaminal epidural steroid injection and needle placement. Image from personal library
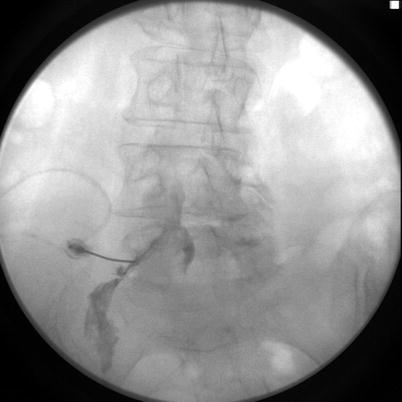
Fig. 15.2
Contrast spread in anteroposterior view showing contrast material spread primarily on the left. Image from personal library
At this point, her differential diagnosis was broad and confounded by her preexisting right-sided weakness and dementia. The differential included weakness along her nerve root secondary to local anesthetic, nerve injury, accidental dural puncture, intrathecal injection, epidural hematoma, anterior spinal cord syndrome, and communication breakdown from dementia. The patient was admitted to the intensive care unit for frequent neurological exams, spine imaging, coagulation tests, and neurosurgical evaluation.
Given her preexisting weakness of her right lower extremity, it was unclear whether the new weakness was unilateral or bilateral. Thus, emergent MRI of the lumbar spine was conducted, which revealed normal anatomy. There was no evidence of either epidural hematoma or spinal cord ischemia. The patient’s symptoms began to resolve within 2 h, and she was discharged home the following day with a diagnosis of weakness due to nerve root injection and baseline preexisting weakness in her right lower extremity.
15.2 Case Discussion
15.2.1 Complications of Transforaminal Epidural Steroid Injections
Intervertebral disc pathology is a common cause of back pain and is often treated with an epidural steroid injection. Steroid can be injected into the epidural space via the interlaminar, caudal, or transforaminal approach. Transforaminal epidural steroid injection (TFESI) is unique among the techniques in that it allows for the delivery of medication into the anterior epidural region [1]. It has been demonstrated that lower dosages of medication are effective when using the TFESI approach [2]. While the reduction of amount of medication injected decreases some risk, the transforaminal approach to the epidural space comes with its own unique risks. These include minor complications like headache and nausea or major complications such as motor weakness, nerve injury, accidental dural puncture, intrathecal injection, discitis, paraplegia, and death.
15.2.2 Anatomical Congestion in the Foramen
The epidural space is found throughout the length of the spinal canal surrounding the outermost meningeal layer, the dura mater. The superior margin is at the foramen magnum, and the sacrococcygeal membrane is the inferior extent of the space. Anteriorly the epidural space is bounded by the posterior longitudinal ligament and posteriorly by the ligamentum flavum and vertebral laminae . Part of the lateral extent is defined by the vertebral pedicles; however, the transition between the epidural space and the paravertebral space is not well defined, and there is no current consensus [3]. In 1981, Crock [4] described it as a single sagittal slice through the narrowest portion of the nerve root canal, and in 1988 Lee et al. [5] divided the foramen into three zones: lateral recess zone, midzone, and the exit zone. More recently, Gilchrist et al. have taken a more comprehensive approach, with detailed description of the anatomic boundaries moving from the medial aspect of the pedicles laterally to the psoas fascia [6].
The anterior and posterior spinal nerve roots that make up each spinal nerve exit the spinal cord cephalad to the intervertebral foramen through which they exit the spinal column. They enter the intervertebral foramen in close proximity to the inferomedial aspect of the superior vertebral pedicle [7]. As they course between the superior and inferior pedicles, the nerve roots coalesce into a single structure known as the spinal nerve . There is an enlargement of the dorsal root just proximal to the origin of the spinal nerve. This enlargement is the dorsal root ganglion (DRG) and is the location of the cell bodies of the sensory nerves [6]. The location of the DRG within the foramen can vary, though at the lumbar levels, they are most often located within the anatomic boundaries of the intervertebral foramen, directly beneath the cephalad pedicle [8, 9]. The DRG of the S1 nerve root is unique however, in that it is often located intraspinally [9].
There are multiple proposed methods of injury to the segmental arteries using the transforaminal approach. They include embolization of particulate medication, direct injury, muscle spasm, compression, intimal flaps, and arterial transection. The artery of Adamkiewicz , also known as the arteria radicularis magna , is the largest anterior segmental medullary artery. It is important as it supplies a majority of blood flow to the anterior spinal cord in the lumbar region through the anterior spinal artery. Most often the artery enters the spinal canal through the intervertebral foramen in an anterior-superior location with respect to the dural root sleeve. The vertebral level and side at which it is found is highly variable. There are conflicting data from the various anatomical studies. There is an agreement that the artery of Adamkiewicz most often occurs on the left side. However, there is a wide range of incidence in the literature, with studies concluding that the range is from 63 to 85% of the time [10, 11]. While the artery has been found as high as T5 and as low as S1, one cadaver study found the artery arose between T9 and T12 in 75% of cases [12, 13]. However, another study found that the artery arose between T12 and L3 in 84% of cases [14]. In an angiography study, the artery was found at T8–L2 in 95.4% of cases, usually from the left T11 level [15]. In one study, the artery was found between T5 and T8 in 15% of cases [13].
In addition, within the intervertebral foramen, there exist venous communications between the internal and external venous plexuses. These major venous structures are most typically found at the inferior aspect of the foramen, in a space formed by internal ligaments [16].
Apart from the spinal nerves, there are other smaller nerves traversing the intervertebral foramen. These are known as the meningeal branches of the spinal nerves, recurrent meningeal nerves, sinuvertebral nerves, or recurrent nerves of Luschka. They branch from the spinal nerve near the origin of the anterior and posterior rami and then reenter the intervertebral foramen [17]. These nerves provide innervation to the facet joints, the annulus fibrosus of the intervertebral disc, and the ligaments and periosteum of the spinal canal. They are typically found in the three o’clock position of the foramen when viewed laterally [16].
As the paired segmental nerve roots diverge from the spinal cord, they penetrate the dura as they course through the intervertebral foramen. They take with them an extension of dura and arachnoid mater, which is known as the dural sleeve . This is anatomically important when performing a TFESI as it can be the route through which medication is aberrantly delivered into the intrathecal space. The dural sleeve ends at the point when the dura becomes the epineurium of the respective spinal nerve. This extension of dura results in a potential space with a nerve surrounded by cerebrospinal fluid [18]. The widest portion of this potential space is found near the dorsal root [19]. The arachnoid mater splits from the dura and ends at about the level of the ganglion, while the dura extends slightly further laterally [19].
The incidence of dural puncture while performing TFESI is unknown. In fact, one survey involving 322 TFESIs did not identify any [20]. Because they are so rare, the most common presenting symptoms are not well defined. However, immediate signs of intrathecal injection include weakness, numbness, persistent paresthesia, respiratory depression, and unconsciousness. Intrathecal injections can also eventually lead to arachnoiditis and meningitis from chemical irritation from the injectate [19].
In contrast to intrathecal injection, subdural injection , or injection between the dura and arachnoid mater, can be difficult to diagnose. Contrast spread is irregular and different than the honeycomb appearance typically seen with epidural spread. Also, the symptoms of weakness appear more slowly than the immediate weakness seen with an intrathecal injection.
Identification of appropriate contrast spread is one of the best ways to avoid subdural injection, which can occur with proper fluoroscopic needle placement or without CSF return on aspiration. Some authors recommend live fluoroscopic injection of contrast media, which they believe that it allows the interventionalist to better identify intrathecal runoff and vascular injection. The typical intrathecal contrast image is described as flat and glasslike and is located in the central canal. This is opposed to the transforaminal epidural contrast spread, which has a honeycomb appearance at the ipsilateral side of the injection. Epidural contrast will spread along the medial wall of the pedicle and out of the foramen, extending past where the dural sleeve ends.
A complication of accidental intradiscal needle placement can be discitis . The incidence of discitis after TFESI is not well known, though there have been published case reports [21]. A study of the rate of discitis after cervical discography demonstrated the incidence to be around 0.44% [22]. The rate of discitis after accidental annulus puncture during TFESI would most likely be less than this, especially if the puncture is identified prior to injection. Some have proposed the use of prophylactic intravenous or intradiscal antibiotics to prevent discitis after disc puncture; however, the available clinical evidence currently does not support regular use of this practice [23].
Arguably, the most devastating complication of TFESI is paraplegia . There have been eight cases reported in the literature [24]. The similarities between the cases were that a particulate steroid was used, rapid onset of symptoms, and MRI evidence of distal spinal cord infarct [24]. It is commonly believed that the etiology is injection of the particulate steroid into one of the following arteries: (a) radicular artery accompanying the nerve root, (b) ascending cervical artery, (c) deep cervical artery, (d) artery of Adamkiewicz, and (e) vertebral artery (this implies faulty technique) [25]. In the cases, the immediate paraplegia was often attributed to the local anesthetic component of the injectate, and thus the diagnosis of spinal cord infarct was delayed until several hours later. The level and laterality of injection varied in the cases, though, of this small sample, it occurred more often on the left side. Of the eight reported cases, six occurred when needle entry was on the left side [24, 26–29]. One case was reported involving each of the following: T12-L1, L1-L2, L2-L3, and S1 [26, 28, 29]. Four cases were described involving L3-4 [24, 27]. As mentioned before, in all cases a particulate steroid was used. Particles in betamethasone, methylprednisolone, and triamcinolone have been shown to be larger than red blood cells [28]. It has been proposed that these particles act as emboli when injected intravascularly and occlude arterioles and meta-arterioles. Dexamethasone has no particles and has not been implicated in any cases of TFESI paraplegia [25].
Some propose using non-particulate steroids to avoid this devastating complication of TFESI. The largest opposition from this however comes from the idea that non-particulate steroids are cleared from the epidural space too quickly for them to have a sustained anti-inflammatory response. There have been limited studies to date that compare the effectiveness of non-particulate to particulate steroids in lumbar transforaminal injections. One study, recently published, examined particulate (triamcinolone) vs. non-particulate (dexamethasone) in 162 patients undergoing lumbar epidural steroid injections. The study found significantly better and more sustained relief with the particulate steroid group [30]. Other recent studies however showed equal efficacy between particulate and non-particulate steroids [31, 32].
Avoiding intra-arterial injection altogether is the best way to prevent paraplegia. Aspiration is the most frequently used method; however, the sensitivity was shown to be only 45% in one study [33]. Three of the eight reported cases of paraplegia documented a negative aspiration [24, 26, 27].Some argue that CT guidance is safest, as soft tissue structures within the foramen can be identified. CT guidance cannot, however, demonstrate arterial uptake of contrast, and there has been a report of paraplegia when the injection was performed under CT guidance [34]. Continuous fluoroscopy is the only method for identifying intra-arterial flow away from needle tip [24].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






