Fig. 41.1
This EEG recording represents left (channel 1) and right (channel 2) frontotemporal trends of density spectral array (DSA) frequency and total power amplitude trends. Recording began following anesthesia induction and continued until 15 min after discontinuation of CPB. Signal loss from 8:55 to 9:25 was the result of electrocautery interference. Note the sudden bilateral loss of all high frequency EEG activity and total power decline at 11:10, immediately after CPB discontinuation. Also observe the rapid return of these measures following successful identification and correction of a potentially injurious physiologic imbalance
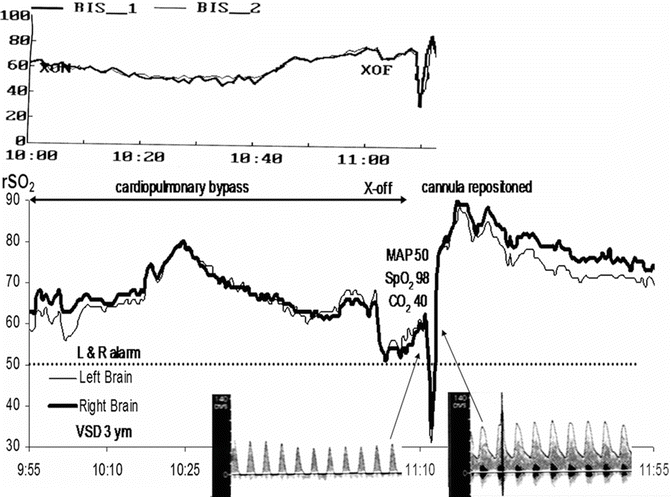
Fig. 41.2
Displayed are the multimodality neuromonitoring changes associated with developing physiologic imbalance and its prompt correction. The upper traces depicting a precipitous bilateral EEG Bispectral Index (BIS) suppression signify diffuse major cerebrocortical synaptic suppression of uncertain origin. Profound bilateral brain regional O2 saturation (rSO2) decline suggests developing brain O2 debt as contributing to the EEG suppression. The suppressed TCD waveform in the lower left panel identifies the cause of the O2 debt as an arterial inflow compromise. Repositioning of the aortic cannula resulted in an immediate recovery of all these measures
This major cortical synaptic suppression was accompanied by growing oxygen debt within the microcirculation of the same cortical regions. The debt was manifested by a precipitous bilateral decrease in rSO2 (Fig. 41.2, middle traces).
Lastly, TCD systolic and diastolic velocities fell from 100/40 to 50/0 cm/s (Fig. 41.2, bottom left waveform). Once the cause of the neuromonitoring changes was identified and corrected, all measures rapidly returned to their pre-insult appearance (Fig. 41.2).
Differential Diagnosis
Technical
The most important advantage of multimodality neuromonitoring is that it minimizes the possibility of misinterpreting a technical recording problem as a sign of nervous system injury. Thus, it is extraordinarily unlikely that a single technical problem would simultaneously produce apparent EEG suppression, brain oxygen desaturation, and diminished blood flow velocity. For example, EEG and TCD signals may be altered or suppressed by electrocautery and other sources of radiofrequency energy (Fig. 41.1). In contrast, because rSO2 is based on near-infrared spectroscopy, it is typically immune to this interference. TCD is susceptible to environmental acoustic influences and both TCD and EEG may be altered by patient movement, but rSO2 remains unaffected. Intense electromechanical radiation within the visible or near-infrared frequency spectra may disrupt rSO2 monitoring but have no effect on the other modalities. Technical problems sufficient to affect all three modalities (i.e., electrical power fluctuations) would almost certainly also disrupt other monitoring and patient support devices.
Physiologic
EEG suppression unequivocally indicates a reduction in cortical synaptic activity, but the cause is often initially undefined. Suppression may reflect a relatively benign process, like excess hypnosis or hypothermia, or a malignant one such as hypoxia or ischemia. Because of this uncertainty, it was imperative that the cause of sudden EEG suppression be promptly identified.
In support of this notion, our group earlier examined the consequences of major EEG suppression in a cohort of 600 adult cardiac surgery patients managed with CPB [14]. EEG suppression was defined as a greater than 50 % decline in total power in at least 1 of 19 channels that persisted for longer than 10 min and was unrelated to either cooling or increased hypnosis. Twenty of the 22 patients awakening with new neurologic deficits experienced noteworthy intraoperative EEG suppression. There also were ten patients with comparable EEG changes but no postoperative neurodeficit. The odds ratio that a neurodeficit would be preceded by EEG suppression was 568:1 (P < 0.001). All the deficit-related EEG changes were widespread, involving frontotemporal cortex in at least one hemisphere. This experience also suggested that the vast majority of ischemia-related EEG changes could be identified using a simplified two- or four-channel electrode montage.
The sudden large decline in rSO2 indicated developing brain O2 debt, but neither its cause nor functional significance could be defined without additional information. In both adult [17] and pediatric [18] patients, low brain rSO2 has been associated with signs of brain injury. However, in the absence of cooling or hypnotic increase, the combination of rSO2 decline with EEG suppression reliably identifies a potentially injurious functionally significant brain O2 imbalance [19].
TCD measures blood flow velocity, not flow [20]. Altered rheology (i.e., hemodilution, hemoconcentration, hypothermia) may thus affect velocity, while total erythrocytic flow remains unaltered. In addition, an abrupt reduction in both systolic and diastolic middle cerebral artery flow velocity may be due to either an actual blood flow decrease or an unrecognized subtle shift in ultrasound probe position. Nevertheless, in the absence of ultrasound probe movement, sudden velocity declines are highly correlated with hypoperfusion [20]. As with rSO2 changes, the functional significance of a velocity decrease requires information on cortical synaptic activity (i.e., EEG).
TCD is very helpful for the causal determination of coupled EEG and rSO2 declines. Ultrasound provides the only means to unambiguously detect a cerebral particulate or gaseous embolic shower [21]. In the present case, a lack of emboliform high-intensity transient ultrasonic signals and an unchanged TCD velocity trend suggested that neither embolization nor hypoperfusion was involved. Attention was then focused on impaired oxygen delivery.
During pre- and postbypass pulsatile perfusion, with stable systemic perfusion pressure and cardiac output, the nature of TCD waveform changes permits discrimination between impaired cerebral arterial inflow and venous outflow. In the former case, both systolic and diastolic velocities fall [22], while in the latter only diastolic velocity decreases and the waveform becomes hyperpulsatile [23]. Sudden onset hyperpulsatility is suggestive of a venous cannula malposition, whereas a progressive onset is characteristic of expanding cerebral edema and intracranial hypertension [22].
Marked cerebral arteriolar constriction resulting from severe hypocapnia could produce all the neuromonitoring changes observed in this case. This explanation was discarded because hypocapnia also would have been manifested by low end-tidal CO2 values.
Pharmacologic
Only severe cerebral vasoconstriction could conceivably lead to total EEG suppression, cerebral hypoperfusion, and oxygen debt. However, vasoconstrictor agents, typically used during cardiac surgery, act on both the systemic and cerebral circulations. Consequently, doses sufficiently high to constrict cerebral arterioles would also result in systemic hypertension. Thus, a pharmacologic etiology for the selective cerebral hypoperfusion and dysoxygenation appeared to be very unlikely.
Surgical
The sudden appearance of severe diffuse cerebral ischemia without systemic manifestations of hypoperfusion immediately directed attention to possible disruption of arterial inflow or venous outflow. In our experience, perfusion cannula malposition is an uncommon cause of cerebral ischemia during adult CPB [24]. However, the probability of its occurrence appears to be inversely related to patient size [14]. We have observed that nearly one-quarter of noteworthy neuromonitoring changes occurring during pediatric CPB are related to malposition of a perfusion cannula, vascular clamp, ligature, or vent [25].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








