Diane C. Seibert, Diane Pace
Menopause
Like the onset of menstruation (menarche), menopause is a normal life event that every woman will experience if she lives past age 50. Unlike the onset of puberty, in many women the hormonal and physiologic changes associated with menopause are superimposed on disorders that manifest primarily in older adults, many of which can be traced to a complex mix of genetic predisposition, environmental exposures, health care access, and long-standing lifestyle choices. During midlife, many women experience significant psychological and social changes; adult children may begin families of their own, and aging parents may need help and support, all of which can adversely affect physical, emotional, social, and financial well-being. To optimize quality of life and health outcomes, providers need to consider all of these factors when developing an individualized care plan for women at or after midlife.
Definition and Epidemiology
Menopause is the final step in a series of reproductive stages in a woman’s life that began decades earlier when she was a 4-week old embryo. At that time, several primordial germ cells (PGCs) split off from the female embryo and begin dividing as they migrated through the embryonic gut, eventually finding their way to the ovary at approximately 16 weeks’ gestation. Unlike somatic cells, which form the rest of the human body, PGCs have the unique ability to divide by both mitosis (up to 5 million copies are made of the initial PGCs) and later by meiosis, wherein the cells are “frozen” in prophase 1 (dictyotene), entering a form of suspended animation that may last years or even decades, until ovulation.1 At birth, a female infant will have approximately 2 million viable oocytes because 1 million will already have degraded. By the time she has her first menstrual period, the number of viable oocytes will have dropped to around 500,000, and she will enter menopause when the final oocyte degrades and disappears, typically at age 52.5. Atresia (cell death and degeneration) is responsible for the loss of almost all of the ova that disappear during a woman’s life. Although the rate varies from woman to woman, the decline in the number as well as the quality of follicles is linear until approximately age 37, when atresia accelerates until menopause.2
As the number of functional ovarian follicles declines, levels of inhibin B fall and follicle-stimulating hormone (FSH) rises, which for a time sustains both follicular development and ovulatory function. The first clinically measurable sign of perimenopause therefore is a high FSH concentration (>10 IU/L) during the early follicular phase (days 2 to 5 of the menstrual cycle). Higher FSH levels may recruit relatively more follicles per cycle, possibly contributing to the acceleration of follicular atresia.3 Overproduction of estradiol by this large cohort of recruited follicles may be responsible for many perimenopausal symptoms, including bloating, irritability, mastalgia, menorrhagia, uterine fibroid growth, vasomotor symptoms (VMSs), insomnia, migraines, and premenstrual syndrome (PMS) dysphoria.4 Although rare, pregnancy is still possible late in the perimenopausal period and women are at risk for unplanned pregnancy until they have been amenorrheic for more than 1 year. Several terms associated with menopause are defined in Table 165-1.
TABLE 165-1
Terms Related to Menopause
| Term | Definition |
| Menopause | The permanent decline in gonadal hormone levels confirmed by 12 months of amenorrhea (12 months after final menstrual period [FMP]) in women with a uterus. The diagnosis can be established using other criteria including history of bilateral oophorectomy, symptoms, and/or serial measurement of endocrine markers. |
| Premenopause | The phase of life that precedes menopause. |
| Premenopausal | Relating to premenopause. |
| Postmenopause | The phase of life after menopause. The 2010 U.S. Census Bureau reported that nearly 40 million U.S. women were older than 55 years, past the age of natural menopause, which occurs at approximately ages 51-52 years years in the United States. |
| Postmenopausal | Relating to postmenopause. |
| Menopausal transition | Begins with the onset of intermenstrual cycle irregularities and/or other menopause-related symptoms and extends through menopause. |
| Perimenopause (sometimes called climacteric) | A clinically useful term that encompasses the most symptomatic years. Perimenopause begins with the onset of intermenstrual cycle irregularities and/or other menopause-related symptoms and extends beyond menopause to include the 12 months after menopause, thus lasting 1 year longer than the menopausal transition. |
| Early menopause, late menopause | Vague terms that have been used to describe menopause that occurs earlier or later than the normal range of menopause. |
| Premature menopause | Menopause that occurs before age 40. Approximately 1% of U.S. women experience premature natural menopause, so of the 49 million U.S. women who were projected to be age 15 to 44 years in 2015, approximately 490,000 would have experienced premature natural menopause.6 |
| Primary ovarian insufficiency or failure | Hypergonadotropic hypogonadism in a woman younger than age 40 years. |
| Induced menopause | Cessation of menstruation after either surgical removal of both ovaries (the most common cause) or iatrogenic ablation of ovarian function (by chemotherapy or pelvic radiation therapy). |
| Premature ovarian failure | The North American Menopause Society as well as the American Congress of Obstetricians and Gynecologists recommend that this term no longer be used. |
The mean age at menarche in the United States has dropped steadily from the early 1800s and continues to decline in some populations,5 but the age of onset of menopause has remained relatively stable for generations. The Study of Women’s Health Across the Nation (SWAN) trial, which followed over 3000 women from multiple states and racial and ethnic groups, found that menopause occurred at age 52.54 years irrespective of racial or ethnic group, age of menarche, or number of lifetime pregnancies.7 Some health and socioeconomic factors, such as self-rated health, higher body weight, lower physical activity levels, negative smoking history, prior oral contraceptive use, higher education level, and employment, were significantly associated with a later onset of menopause, suggesting that the onset of menopause is influenced by a number of factors, perhaps explaining some of the relationship between reduced morbidity and mortality in women who enter menopause later in life.2
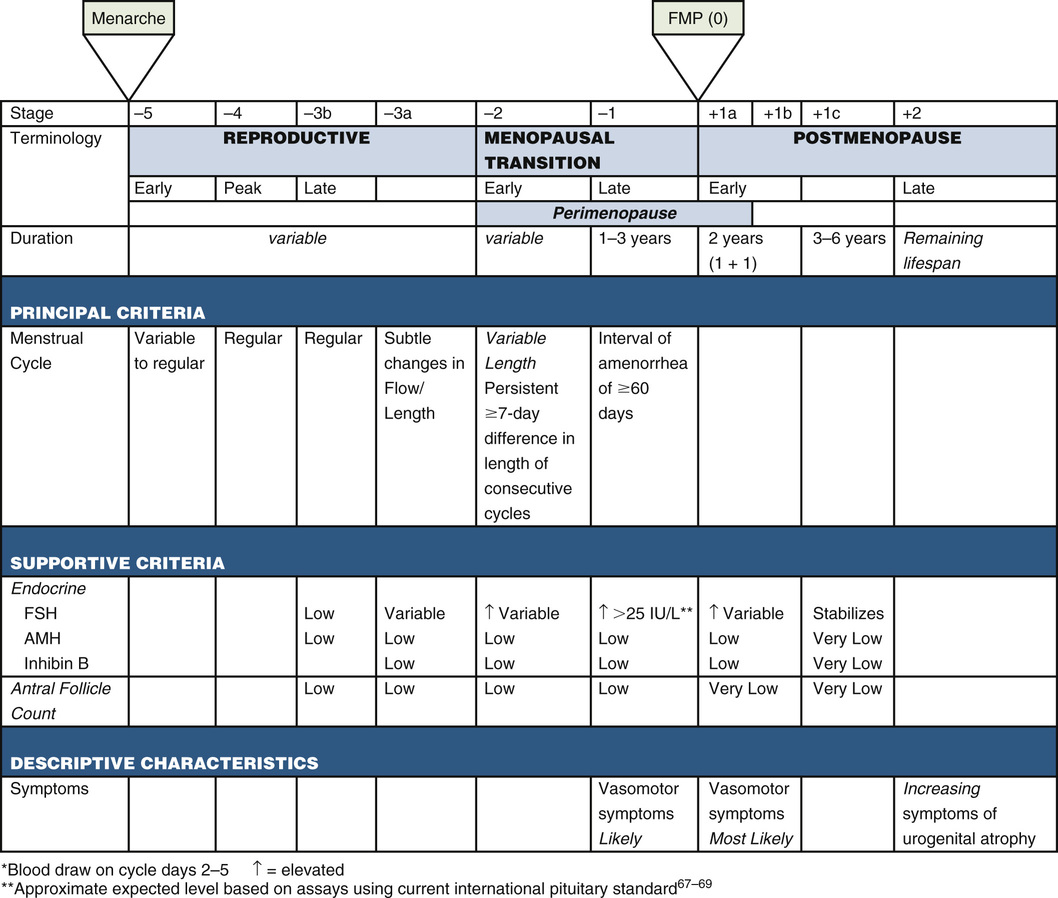
Before 2001, there was no clear definition of menopause, nor was there a staging system to clearly communicate the physiologic changes occurring during the final 10 to 15 years of a woman’s reproductive life. Since that time, the Stages of Reproductive Aging Workshop (STRAW) has met twice, once in 2001 and again in 2011 (STRAW +10), to develop a clear and simple set of definitions and descriptions to simplify the criteria for menopausal bleeding.8 Consistent use of the STRAW +10 criteria holds the promise of improving clinical decision-making by clearly articulating where a woman is along the continuum, and supporting research efforts because it offers a structure within which to compare studies conducted on women in midlife (Fig. 165-1).
The STRAW +10 criteria divide the perimenopause, menopause, and postmenopause periods into seven phases, five of which occur before the final menstrual period (FMP) and two of which occur after the FMP. As with every other developmental phase in life, individual variability is the norm, with some women moving rapidly through one or more stages, some skipping stages altogether, and some shifting back and forth between stages. In general, the transition through these 7 phases is predictable, but chronologic age does not accurately predict reproductive ability, so menopause should be included in the differential diagnosis whenever a woman reports menopausal symptoms. Because most American women will spend nearly one third of their lives in the postmenopausal period, the implications for women, clinicians, and the health care system are huge.
Physiology
Reproduction is controlled by a highly complex series of interactions among a number of different hormones. A complete discussion of all of them is beyond the scope of this chapter, but the structure and function of a few key hormones (estrogen, progestogen, androgens) are discussed here to provide a frame of reference for the management section, later.
In the early 1970s, estrogen’s effects were believed to be primarily reproductive, having little effect on tissues outside the uterus and mammary glands. Over the past 40 years, however, evidence has revealed that estrogen exerts its effects on many organs. At least two distinct estrogen receptors (ER-α and ER-β) have been identified, both of which are present in ovarian and central nervous system (CNS) tissues. In other body tissues, however, one form or the other predominates. For example, ER-α is found in hepatic, uterine, and breast tissue, whereas ER-β is found in bone, blood vessels, lungs, and urogenital tissues.9 Further complicating the estrogen picture, there are three known forms of human estrogens: estrone (E1), estradiol (E2), and estriol (E3). E1, the least abundant, is derived from stored body fat and is the primary estrogen in postmenopausal women. E2, produced by the ovarian follicle during the reproductive years, is the most potent and abundant (10% to 29%). E3, the least potent, predominates during pregnancy. Serum estradiol levels vary widely during the menstrual cycle—below 10 pg/mL early in the follicular phase, then rising above 800 pg/mL at midcycle, then dropping back down to 200 to 340 pg/mL during the luteal phase. More than 95% of the circulating E2 is produced by the dominant follicle, and less than 5% is derived from peripheral conversion of E1.9
Progesterone, secreted during the luteal phase of the menstrual cycle, first appears right after ovulation and rises steadily for about 10 days before dropping back down to baseline if no pregnancy occurs. Normal progesterone levels range from 2 to 20 ng/mL depending on the day the level is drawn.10
There are five clinically important androgens in women: testosterone, dihydrotestosterone (DHT), androstenedione, dehydroepiandrosterone (DHEA), and dehydroepiandrosterone sulfate (DHEAS). Although often referred to as androgens, androstenedione, DHEAS, and DHEA are actually prohormones that are converted to active androgens in body tissues. DHEA in particular plays an important role in the production of ovarian testosterone. In reproductive-age women, a slight but significant rise in serum testosterone is measurable immediately before ovulation. Evaluating serum androgen levels can be challenging for several reasons. First, only 1% to 2% of testosterone is free to circulate because two thirds is bound to sex hormone–binding globulin (SHBG) and the remainder is bound to albumin, so changes in SHBG production or albumin levels can affect the amount of testosterone available to tissues.11 Second, serum androgen levels do not accurately reflect the amount of androgens available to androgen-dependent or androgen-sensitive tissues such as the skin, the clitoris, or the vulva, because the ratio of plasma DHT levels to serum testosterone levels is low (0.3:1) whereas the ratio is much higher (2:1) in tissues where conversion from DHT to testosterone is actively occurring.12
Antimüllerian hormone (AMH) produced by the follicular granulosa cells plays an important role in follicle recruitment and selection and has recently been found to be a useful marker of ovarian reserve. After rising in adolescence and early adulthood, AMH levels gradually decline, becoming undetectable approximately 5 years before the FMP.13 To date, AMH levels have primarily been used in infertility settings, but serial AMH levels may soon be used to predict the age of menopause.
The first sign that a woman is entering perimenopause (stage −3a) might be (but is not always) menstrual cycle changes. In response to declining ovarian reserve, inhibin B levels drop, and FSH levels rise to more aggressively stimulate the remaining follicles, and although the luteal phase of the cycle remains 14 days, the follicular phase shortens by 2 to 4 days, causing the overall cycle to become shorter (i.e., 25 to 26 days rather than 28 days).8 The STRAW +10 staging illustrates this because stages −2 and −1 are characterized by significant menstrual cycle irregularity, reflecting the increasingly wide swings in hormonal levels and the increasingly frequent anovulatory cycles.
Although the FMP marks the onset of menopause, it is difficult to tell clinically when that FMP has occurred. No serum marker sensitive enough to definitively mark this transition has yet been identified, and a single assessment of several hormone levels (FSH, luteinizing hormone [LH], and estradiol, for example) is unreliable when trying to determine when the FMP has occurred. Recent large studies such as SWAN have found that using a combination of factors such as comparing E2 levels with a level drawn earlier in the perimenopausal period, race or ethnicity, and timing of the serum collection (early follicular phase) is a better predictor of the probability of a woman having had her FMP.14 It is important to remember that although the STRAW +10 criterion of one episode of more than 60 consecutive days of amenorrhea is enough to consider a women older than 45 years in late menopausal transition, it is unreliable in women younger than 40 years who have prolonged (>120 days) episodes of amenorrhea, even when their FSH levels are in the menopausal range. These women should be evaluated for other causes of amenorrhea because once treated, many will resume regular menstruation and have had successful spontaneous pregnancies.8
Research and Clinical Trials
The Coronary Drug Project (1966 to 1975) was the first clinical trial to examine whether estrogen would reduce mortality in men with a history of myocardial infarction (MI).15 A total of 8341 men ages 30 to 64 participated in the study. They were randomly assigned to one of six treatment arms: 2.5 mg of conjugated equine estrogen (CEE) per day, 5.0 mg of CEE per day, 1.8 g of clofibrate per day, 6.0 mg of dextrothyroxine sodium per day, 3.0 g of niacin per day, or 3.8 g of lactose placebo per day. It is important to note that the doses (2.5 and 5 mg) of CEE are extremely high compared with doses (0.325 to 0.625 mg) prescribed today, and that CEE contains several different estrogenic compounds. The primary estrogen in CEE (>50%) is estrone, but the drug also contains equilin (15% to 25%), sodium sulfate conjugates, 17α-dihydroequilin, 17α-estradiol, and 17β-dihydroequilin—all target ERs in different body tissues. The estrogen arm was stopped prematurely because men taking 5 mg CEE had an early excess of heart attacks, thromboembolic events, and estrogenic symptoms, and men taking 2.5 mg CEE were experiencing no benefits. The study concluded that high-dose estrogen is not good for men, but it did not advance our understanding of the effects of estrogen in women.15
The first randomized prospective controlled trial examining the effects of estrogen in postmenopausal women began in 1965 and continued for 22 years. In this study, Nachtigall and her team randomized 84 matched pairs of chronically hospitalized women to placebo or hormone therapy (HT), exploring a wide array of clinical end points (MI, cancer, thromboembolism, gallbladder disease). During the first 10 years, the women were randomized to placebo or CEE 2.5 mg and medroxyprogesterone acetate (MPA) 10 mg, but during the final 12 years of the trial, the CEE dose was decreased to 0.625 mg and the women were permitted to stop or to switch groups. Overall, Nachtigall and her colleagues found that women taking CEE had a higher incidence of gallbladder disease, but there were no differences in MI, endometrial cancer, or thrombophlebitis between groups. Women taking hormones were found to have a decreased incidence of breast cancer (no cases in the HT group versus six cases in the placebo group).16
Since those early days, many large studies have been conducted exploring the impact of postmenopausal hormones on a variety of clinical end points, particularly cardiovascular health, osteoporosis, breast cancer, and colorectal cancer (CRC). The majority of the earlier observational and randomized clinical trials and meta-analyses found that standard-dose estrogen-alone HT decreased coronary heart disease (CHD) and all-cause mortality in women who were younger than age 60 and recently (within 10 years) menopausal. The data on combined estrogen-progestogen HT were not as compelling, however. Most studies examining the effect of combined HT on a number of different health outcomes did not find a significant increase or decrease in CHD.17 Two large randomized controlled trials, the Heart and Estrogen/Progestin Replacement Study (HERS) and the Women’s Health Initiative (WHI), were launched in the late 1990s to find answers to important questions about the impact of HT on the cardiovascular system.
HERS, a two-phase, randomized, blinded, placebo-controlled trial of continuous-combined estrogen-progestogen (refers to either progesterone or progestin) therapy (EPT) in postmenopausal women, was designed to determine whether combined EPT (CEE plus MPA) altered the number of cardiovascular disease (CVD) events in postmenopausal women with established CHD. The study was almost terminated early because women in the treatment arm (receiving HT) experienced more cardiac events during the first year of hormone use than the placebo group did. After the first year, however, this difference in risk reversed and women receiving HT were found to have experienced slightly fewer CVD events overall. To see whether this trend toward fewer CVD events continued, the study was extended for 3 additional years (HERS II), but no continuing CVD benefit was found. The HERS study clearly demonstrated that HT does not provide CVD protection in women with established heart disease, and during the first year of HT use, women with existing heart disease were at increased risk for cardiac events. The authors postulated that the estrogen’s prothrombotic, arrhythmic, and ischemic effects during the first year counteracted any improvement in lipids.18
The WHI was a large multicenter, multiyear trial consisting of three interrelated randomized, blinded, placebo-controlled hormone trials and an observational study designed to examine the risks and benefits of HT in healthy postmenopausal women aged 50 to 79. Women with a uterus were assigned to the EPT arm and women without a uterus were assigned to the estrogen therapy (ET)–only arm. Participants were then randomly assigned to receive either the appropriate HT or a placebo. Before the study began, researchers identified two major clinical outcomes (CVD and invasive breast cancer), six minor outcomes (stroke, pulmonary embolism, endometrial cancer, CRC, hip fracture, and death from other causes), and a global index (a number reflecting the balance of risks and benefits).19 The EPT arm was terminated 3 years early, in July 2002, because the global index rose above a predetermined risk threshold. Although the absolute risk of harm was very small and there was no difference in the number of deaths between the EPT and placebo groups, the trend toward increasing risk could not be ignored. The ET group continued to the projected end point because the global index threshold was not crossed.
Overall, the WHI demonstrated that the health risks and benefits of HT vary depending on the type of hormones used (estrogen or EPT), the age of the woman, and how remote from menopause a woman is when she begins taking them. Systemic HT has been shown to reduce moderate to severe menopausal symptoms and decrease fracture risk. Although the absolute risk for harm was very low, the WHI clearly showed that both estrogen and EPT increased the risk for developing venous thromboemboli (and associated disorders such as stroke and CVD), although the risk of ischemic stroke was rare in the 50- to 59-year age group.20 The risks and benefits for other health outcomes at different ages varied. After 5 years or more of continuous use of EPT, the study found an increased risk of breast cancer, which decreased after the HT was discontinued. This was not found in the estrogen-alone group even after 7 years of follow-up. Counseling women about the risks and benefits of HT is complex; the evidence shows that although HT is helpful and appropriate for managing menopausal symptoms in some women, HT should not be used for chronic disease prevention. For a detailed summary of the final WHI findings, see the article by Manson.19
Clinical Presentation
The clinical presentation and symptoms of menopause may vary significantly from one woman to another depending on the woman’s age and underlying health status. Some manifestations such as VMSs are clearly associated with menopause, but others such as sexual dysfunction are much more elusive. Management of symptoms in women at midlife must therefore be considered in the context of healthy aging, because age is a confounder for symptoms and diseases that may be associated with or directly affected by menopausal management. The primary driver for the physiologic changes associated with menopause is the dramatic decline in estrogen levels, causing a number of short- and long-term physical changes including cycle irregularity, VMSs (hot flashes), urogenital atrophy (vaginal dryness, urinary incontinence, pelvic floor dysfunction), mood changes, and poor sleep and sexual functioning.
Irregular Bleeding
Abnormal uterine bleeding (AUB) is the most frequently reported perimenopausal symptom, with nearly 90% of women reporting 4 to 8 years of menstrual cycle changes before experiencing their FMP. Although some women experience fewer cycles with lighter bleeding during the menopausal transition, many women seek medical assistance for prolonged or heavy menstrual bleeding (HMB), which can cause significant anemia, avoidance of activities, including sexual intercourse, and a diminished quality of life.21 Early in the menopausal transition, cycle irregularities are caused by disruption of the communications among the hypothalamus, pituitary, and ovaries, but as the FMP approaches, anovulation becomes more common, increasing the risk for unopposed estrogen exposure, endometrial hyperplasia, and cancer. Although most perimenopausal women with heavy or irregular bleeding do not have anatomic pathology, common causes of HMB such as thyroid dysfunction, pituitary adenoma, cervical polyps, uterine fibroids, endometriosis, and endometrial hyperplasia or cancer should be ruled out before attributing the cycle changes to the menopausal transition.
Depending on the clinical presentation, a comprehensive AUB workup could include laboratory tests to rule out pregnancy and sexually transmitted infections, hematologic parameters (complete blood count, liver function, coagulation profile), and serum hormone levels (thyroid, prolactin, FSH, estradiol, progesterone, testosterone, and DHEAS). Depending on these results, additional procedures might include endometrial biopsy and/or dilation and curettage, transvaginal ultrasonography, hysteroscopy, or sonohysterography.
AUB can be managed medically, hormonally, and/or surgically, and treatment should be individualized based on the severity of the AUB, the impact of the bleeding on a woman’s quality of life, her personal preferences and contraceptive needs, and her overall health status. Often the first therapy tried is medical management, which includes nonsteroidal anti-inflammatories, tranexamic acid, and desmopressin (for women with underlying bleeding disorders). Medical management is often combined with hormonal management, which includes gonadotropin-releasing hormone (GnRH) agonists, oral progestogens and contraceptive options such as low-dose oral contraceptive pills, depot MPA, and levonorgestrel-releasing intrauterine devices (IUDs). Referral for consideration of surgical options (endometrial ablation, polypectomy, myomectomy, and/or hysterectomy) is usually reserved for women who have anatomic pathology such as fibroids, endometrial hyperplasia, and cervical disorders such as dysplasia and polyps, and procedures are tailored to treat those specific conditions.21
Vasomotor Symptoms
VMSs, considered the hallmark of the female climacteric, are characterized by vasomotor instability, hot flashes, day sweats, and night sweats. As many as 75% of perimenopausal and postmenopausal women report experiencing VMSs for 6 months to 2 years, although some women will report having hot flashes for more than 10 years. The peak incidence of hot flashes is within 2 years of the FMP,22 but the physiologic mechanisms are still not completely understood. The term hot flash is used to describe the sudden onset of head, neck, and chest flushing, accompanied by a feeling of intense body heat, profuse perspiration, and modest heart rate increases that lasts generally from 1 to 5 minutes. Often as skin temperatures return to normal, there are decreases in core body temperatures with significant heat loss resulting in chills for some women. According to the SWAN study, the prevalence of hot flashes differs across U.S. racial and ethnic groups. Within this study of over 15,000 women, black women reported VMSs most frequently, followed by Hispanic, white, Chinese, and Japanese women.22 Other variables for increasing VMS include obesity, low socioeconomic status, and surgically induced menopause. Factors such as depression, history of depression, anxiety, perceived stress, and poor physical health have also been associated with a higher risk. Higher education levels appear to be somewhat protective, but other factors, such as age, current smoking, alcohol use, and employment status do not appear to be significantly associated with an increased risk for developing severe VMSs.23 Conditions such as thyroid disease, infection, insulinoma, pheochromocytoma, autoimmune disorders, new-onset hypertension, diabetes, and autonomic dysfunction may also cause hot flashes and should be considered if appropriate in the differential diagnosis.22
Fluctuating hormone levels are associated with hot flashes. Although there have been numerous research studies and theories developed regarding the causes of hot flashes, specific mechanisms or brain areas involved in thermogenesis and heat perception are still being explored and more research is needed.22
Mild to moderate hot flashes can often be managed with lifestyle changes, such as getting regular exercise; wearing layers of light clothing; lowering the thermostat; avoiding spicy foods, caffeine, or alcohol; and engaging in relaxation exercises. If medical management is necessary, ET is both the primary indication and most effective treatment for VMSs, and every systemic ET and estrogen-progestogen therapy (EPT) product has received regulatory agency (U.S. Food and Drug Administration [FDA]) approval for vasomotor instability. Estrogen options include systemic estrogen; combination products including combined estrogen-progestogen or estrogen/bazedoxifene (BZA); a selective estrogen receptor modulator (SERM) combined with conjugated estrogen; or combined oral contraceptives (in women requiring contraception).21 Other therapies available for women who either cannot or do not want to take HT include low-dose, 7.5-mg paroxetine (Brisdelle), which received FDA approval for treatment of vasomotor flushes. Some options that do not have FDA approval for this indication include clonidine; and gabapentin. In addition, some antidepressants (e.g., venlafaxine, escitalopram) have been shown to be helpful for VMSs.21 Many herbal or alternative remedies including soy, isoflavone supplements, black cohosh, vitamin E, and omega-3 fatty acids are popular and generally low risk, but none has been proven to be significantly more effective than placebo, and some may be harmful.21 Clinical trials on a new supplement, Relizen, available in Europe for more than 10 years and introduced in the United States for management of VMSs, suggest that it improves symptoms and is an effective nonestrogenic alternative to HT.24 Refer to the North American Menopause Society (NAMS) resources for a list of products and complementary and alternative options.
Genitourinary Syndrome of Menopause
Vulvovaginal Atrophy.
Vulvovaginal atrophy (VVA) refers to changes in the vagina and vulva that develop during the postmenopausal period as a result of declining estrogen levels in these tissues. VVA is a chronic, progressive disorder that often causes significant discomfort and worsens without treatment.25 The vagina may be narrow and less elastic, with thin, pale, dry, or inflamed side walls with petechia. As a result of these changes, women with VVE often report dryness, burning, irritation, vaginal pain, and dyspareunia. These changes may be seen throughout the genitourinary tract. As many as half of midlife and older women may experience urinary symptoms such as frequency, urgency, dysuria, and recurrent urinary tract infections.21
Two large studies—Vaginal Health: Insights, Views and Attitudes (VIVA) and Real Women’s View of Treatment Options for Menopausal Vaginal Changes (REVIVE)—examined the impact of VVA on women, and found that VVA negatively affects women’s lives, lowers their quality of life, and has negative consequences on their sexual health.26,27 The REVIVE survey also noted that women reported that few health care practitioners had had any conversations with them about VVA, prompting the need for clinicians to initiate this conversation with patients before the onset of symptoms, to assess for early clinical changes, and to discuss options for preventing and/or treating this disorder.
Not only did these surveys point out that clinicians are reluctant to initiate discussions about VVA, but medical societies and others have noted that the media and the general public are uncomfortable with using the term vagina in public discourse, and the term atrophy has a negative connotation for many women.25 Given that the term VVA does not include the anatomic areas of the urinary tract that are also affected by estrogen deficiency of menopause, a panel was convened in 2012 to discuss the need for more acceptable terminology to replace VVA. Two large societies, NAMS and the International Society for the Study of Women’s Sexual Health (ISSWSH), acknowledged that a new term was needed that would be more “scientifically accurate, descriptive, inclusive, and socially acceptable.” The term genitourinary syndrome of menopause (GSM) was adopted by both organizations. It is anticipated that this term will be accepted by other professional societies, providers, researchers, educators, women, and the media and will improve communication, research, education, and the treatment in this area of women’s health.25
GSM should be evaluated and interventions individualized for each woman as appropriate based on her symptoms and how these symptoms may be creating distress. Nonhormonal over-the-counter (OTC) lubricants and moisturizer products are often first-line therapies for symptoms of vaginal dryness and to reduce friction on atrophic tissue during sexual activity. Regular sexual activity or nonpenetrative sexual activity through use of oral stimulation or self-stimulation with massage promotes blood flow to the genital area and helps to maintain vaginal health. For symptoms requiring intervention for severe symptoms, including dyspareunia, minimally absorbed vaginal low-dose ET is the treatment of choice for women who have no medical contraindications to its use. Benefits of low-dose vaginal estrogen include restoration of vaginal blood flow, a decrease in vaginal pH, and improvement in the thickness and elasticity of the tissues. The FDA has also approved ospemifene, an oral SERM, for the treatment of moderate to severe dyspareunia associated with VVA.22
Central Nervous System Effects
Mood Changes.
Mood disorders (particularly depression) are nearly twice as common in women as in men. Recent randomized, controlled trials have demonstrated that the hormone shifts associated with menopause can significantly increase the risk for new-onset and recurrent depression. It does not appear to be the estrogen alone, however, because a number of menopausal symptoms are independent risk factors for depression, including VMSs, insomnia, severe premenstrual or postpartum mood swings and depression, stressful life events, history of depression, high body mass index, low socioeconomic status, and hormone or antidepressant use. Estrogen helps modulate several neurotransmitter pathways, particularly serotonin and norepinephrine, helping to regulate mood. Studies have also shown that transdermal estrogen and serotonergic and noradrenergic antidepressants relieve both mood and VMSs.28 If depressive symptoms appear when a woman begins taking HT, the provider should examine the type of HT being used. Progestogens have been shown to worsen mood in some women, particularly those with a history of PMS, premenstrual depressive disorder, or clinical depression.
Insomnia.
Insomnia may occur in association with or independent of hot flashes, and HT has been shown to provide substantial relief for early morning waking, even in women without significant VMSs. The irritability that so often accompanies insomnia also responds positively to HT. Women receiving HT have been shown to have shorter sleep latency and more frequent and prolonged rapid eye movement sleep.
Sexual Functioning.
Decline in sexual activity during the menopausal years is probably influenced more by culture and attitudes than by nature and physiology. Significant determinants of sexual activity for older women are the availability and health status of a sexual partner.29 Data from the PRESIDE (Prevalence of Female Sexual Problems Associated with Distress and Determinants of Treatment Seeking) study point out, however, that sexual problems are reported by approximately 40% of U.S. women and peak in women at midlife.30 Psychological, sociocultural, interpersonal, and biologic factors occurring at midlife also contribute to sexual issues at midlife.22 Changes in mood and well-being and untreated anxiety or depression may have an effect on sexual disorders associated with menopause. Low estrogen levels at menopause often leading to GSM, also have been associated with a decline in sexual function. Although testosterone is an important factor in midlife sexual changes for women, playing a role in motivation, desire, and sexual sensation, an association between decreased androgen levels and impaired female sexual function is not clearly supported in the evidence.22
Taking a sexual history can facilitate a discussion on sexuality and sexual health, addressing the woman’s concerns and identifying any potential issues such as intimate partner violence or risk for acquiring a sexually transmitted infection. The online handout Talking to Patients About Sexuality and Sexual Health published by the Association of Reproductive Health Professionals provides a good reference guide for clinicians.31
Chronic Disease
With rare exceptions, menopause occurs as women enter late midlife, when many of the disorders associated with the aging process emerge. Estrogen has been shown to improve quality of life and to reduce risk for some diseases associated with aging. More recently, estrogen has been shown to increase risk for adverse outcomes from some diseases in some women. The question for clinicians is how to best manage menopausal symptoms while maintaining optimal health in older women. To address these important questions, NAMS has published a series of position statements to help inform and guide clinicians in the management of midlife women.32 In addition, in October 2014 NAMS published its most current guidelines, the North American Menopause Society Recommendations for Clinical Care of Midlife Women.21 This 25-page publication is a wonderful resource for clinicians because it both identifies key points and provides clinical recommendations to assist in optimizing health for older women.
The disorders that are of most concern to clinicians working with menopausal women include CVD (venous thromboembolism [VTE], stroke, and CHD), osteoporosis, atrophic vaginitis, cognitive decline (Alzheimer dementia [AD]), and certain cancers (endometrial, breast, ovarian, lung, and colorectal), all of which are described here. A brief discussion of the disease is followed by what is known about the risks and benefits of HT for that particular condition.
Cardiovascular Disease
Cardiovascular disease is a broad term encompassing three distinct but related disorders: CHD, stroke, and VTE. According to the World Health Organization (WHO), in 2008 more than 7.3 million people died from CHD and another 6.2 million died from a stroke, making CVD responsible for more deaths worldwide than any other condition.33 The classic description of MI (heart attack) often includes chest, left arm, or jaw pain or pressure, but many women are not aware that other, more atypical symptoms such as pain at rest, shortness of breath, nausea, and fatigue may be more common presenting manifestations in women. It is important, therefore, for health care providers to not dismiss a woman’s reports of chest pain, fatigue, or shortness of breath as benign or noncardiac in nature.
Osteoporosis
Osteoporosis is a serious and disabling disease that has become a major public health problem as the American population has aged. A study by the National Osteoporosis Foundation (NOF) estimates that 10.2 million Americans currently have osteoporosis and another 43.4 million have osteopenia. Assuming that disease prevalence is unchanged. It is predicted that within 5 years, the number of Americans affected by this disease will increase from 54 million (nearly half of all adults) to 64.4 million, and that by 2030, 71.2 million people will be affected. Osteoporosis is a major risk factor for fracture, and the NOF estimated that in 2014 approximately 2 million fractures could have been attributed to the disease.34 Osteoporosis is often site specific, affecting the femur, hip, spine, and forearm, but not necessarily uniformly in the same woman; one woman might have osteoporosis of the spine but normal bone density in the femoral neck, whereas another woman may have osteopenia in both areas. As the incidence of osteoporosis increases, so does the incidence of pathologic fracture. Interestingly, after adjusting for age and gender, the relative death rates after fractures sustained in severe trauma were lower than the death rates associated with pathologic fractures sustained after no more than moderate trauma. The estimated 10% overall increase in mortality was just as high regardless of fracture site (excluding hands and feet), and persisted for at least a decade after the initial pathologic fracture.35
The most important factors affecting risk for osteoporosis are gender, age, dietary calcium intake, hormone status, genetics, medication use, and certain disease states. Additional risk factors include low vitamin D levels, excess alcohol intake, sedentary lifestyle, low lean body mass, smoking, and early onset of menopause (Box 165-1). Risk factors specifically applicable to postmenopausal women are discussed here; a more in-depth discussion of osteoporosis risk factors can be found in Chapter 182.