Fig. 15.1
Mannequin-based simulators placed in the larger simulation context which includes an immersive environment, inputs and outputs, and an operator learner pairing
Mannequin-based simulators should be viewed as part of the spectrum of simulation. For clarity, mannequin-based simulators will be assumed to include simulators that have the ability to display a range of physical attributes and physiologic parameters, are life-sized, and have the ability to have controllable inputs that result in outputs. The mannequin-based simulators in this chapter are controlled by a computer platform (Figs. 15.2 and 15.3) which allows for programmability as well as “on the fly” changes and come in four general sizes: adult, child (5–12 years old), infant (2–18 months old), and neonate (0–2 months old). Mannequin-based part-task trainers will also be considered in this chapter.
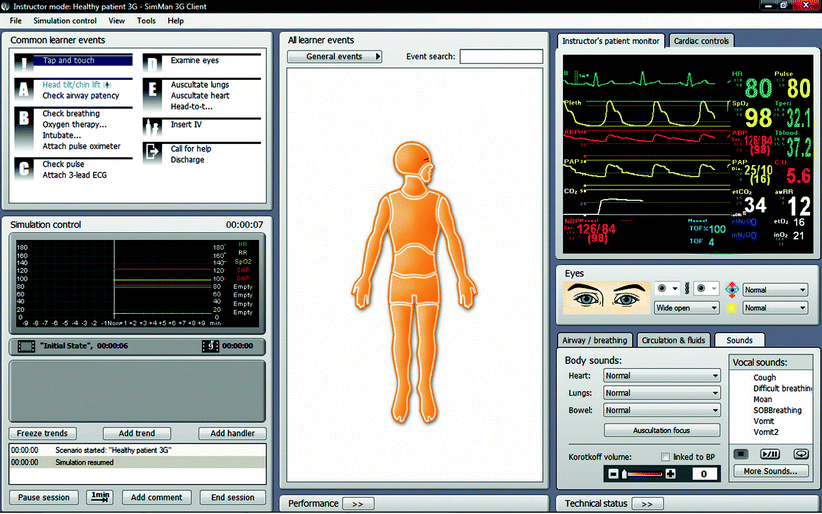
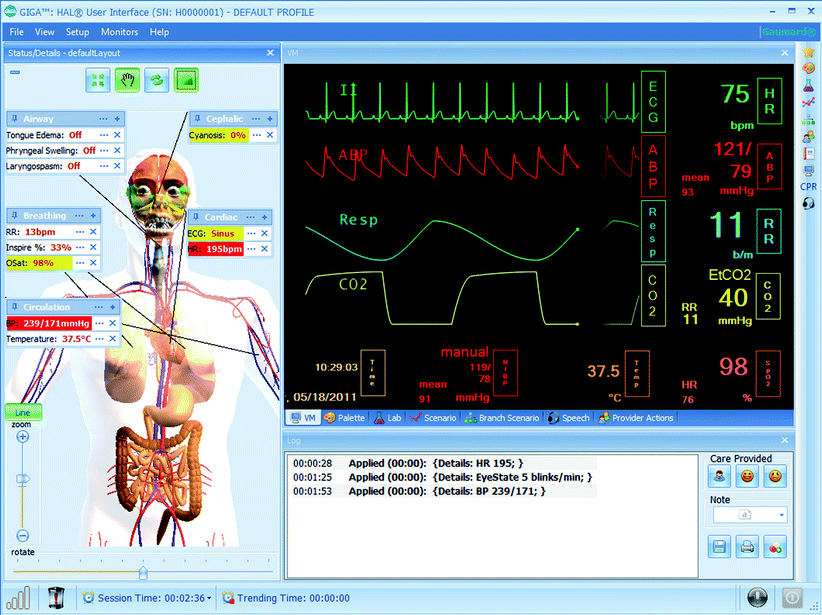

Fig. 15.2
SimMan® 3G user interface (Photo courtesy of Laerdal Medical. All rights reserved)

Fig. 15.3
Gaumard’s GIGA user interface (Photo courtesy of Gaumard Scientific 2012. All rights reserved)
Control and Modeling
Broadly speaking, mannequin simulators are either operator-driven or autonomous. Operator-driven mannequins rely on the instructor, rather than modeling, to drive the simulator. Responses to interventions are controlled by the operator since there is typically limited ability of the mannequin to provide intervention feedback. Autonomous simulators utilize mathematical modeling algorithms to prompt changes in status or physiology based on intervention. For example, giving intravenous fluids to an autonomous simulator will correct signs of hypovolemia automatically (i.e., increased blood pressure with a reduction of heart rate), while the same intervention in an operator-driven mannequin requires changing of the appropriate vital signs by the operator. A learner’s perspective of the simulated scenario is the same regardless of type, but the operator’s ability to control the scenario varies. Operator-driven mannequins are typically less complicated and easy to control but are dependent on the operator to ensure that the physiologic data (e.g., vital signs) are realistic. Realism is less of an issue with autonomous simulators, but the plethora of physiologic parameters that can be manipulated can be overwhelming and generally requires at least a modest understanding of the physiologic mechanisms.
Even though a simulator may be primarily operator-driven, varying degrees of physiologic modeling may be included that allows for autonomous changes. For example, some operator-driven mannequins may respond to the application of oxygen or use of bag-valve mask by increasing the simulator’s oxygen saturation without input from the operator. Additionally, some operator-driven mannequins detect electrical current and can respond to cardioversion, defibrillation, or pacing.
Complex mathematical models are crucial to the development of today’s autonomous mannequin simulators to predict physiological and pharmacological responses. These models first appeared in screen-based simulators before being integrated into mannequin simulators. The models use mathematical terms to describe the relationship between two or more physiologic parameters and behave similarly to real-life processes. The model acts as an “engine” for the simulator and is affected by learner intervention as well as events scripted by the operator. By accurately generating physiologic responses to learner actions, these models can reduce, for instance, the need for operators to specify moment-to-moment vital signs, significantly reducing the operator’s workload to allow more time for observing learner activity.
Not all patient conditions can be easily modeled. Because of inter-patient variability and the large number of contributing factors, there is no way to predict exactly when, for example, a patient will have a cardiac event. A model can, however, calculate the likelihood of such an event based on the information available.
Fidelity
Fidelity refers to the degree a simulator is able to reproduce a real-world environment. Though levels of fidelity are not formally defined, it is generally accepted that the advanced mannequin-based simulators discussed in this chapter are in the higher-fidelity ranges. These “full-scale” or “high-fidelity” simulators are usually found in high-fidelity environments and designed to interact with healthcare providers on both the psychomotor and cognitive domains of learning [1]. Fidelity ultimately depends on the intended application. A mannequin designed for neurologic surgery would not be considered high fidelity in a simulated labor and delivery setting where the objectives focus on techniques of vaginal delivery.
Mannequin features such as realistic heart sounds, palpable pulses, and the ability to speak add to simulation fidelity. Though these advanced features come at a higher cost [2], there are many studies that indicate training with realistic mannequins improves clinical performance (ACLS [3], detection of murmurs [4]), and improves skills with full-mannequin simulators (airway management [5], medical emergencies [6], PALS [7], ACLS [8], trauma management [9]). Conversely, some studies indicate that learners training with higher-fidelity mannequins have no advantage in skills or knowledge compared to learners training with lower-fidelity modalities [10–12]. There appears to be insufficient evidence to make a final determination on how fidelity correlates with effectiveness or clinical outcomes. It is important to note, however, that learners consistently report greater satisfaction with more realistic high-fidelity mannequins [12, 13]. In some regards, the most appropriate level of fidelity depends on the learners and the learning objectives. It is important to note that even the most advanced simulators have important limitations and none rival the sophistication of, for instance, flight simulators used by pilots.
Programming Principles
Commonly the simulation scenario will last approximately one-third of the allotted time allowing two-thirds of the time for debriefing (Chaps. 6 and 7). Depending on the complexity of the case, the types of learners, and the learning objectives, the simulation scenario may only last a matter of minutes. Due to this relatively short time frame, clinical treatments such as administering a fluid bolus, which may typically occur over 5 min, often occur over 1 min or even instantly in simulation. These time-scaling properties are generally built into the commonly available programming platforms. When programming these treatment effects on the simulator and monitor, it is important that they do not occur too fast to be unrealistic but not too slow that the intensity of the session is lost.
All simulators can be run “on the fly,” meaning without a preexisting program. This works very well when very few changes occur or only one change occurs at a time. For example, if a patient develops supraventricular tachycardia (SVT), one can simply change the rhythm from normal sinus rhythm to SVT. This change can be detected on the patient monitor (increase in heart rate and loss of P waves) as well as the simulator (increases in palpable pulse rate and heart sounds). Clinically speaking, however, many changes occur over time and multiple changes may occur simultaneously. For example, a child in shock will have increased heart rate, decreased blood pressure with either a narrowed or widened pulse pressure, increased respiratory rate, and decreased end-tidal carbon dioxide. If this child is given fluids, there will be multiple simultaneous changes over a period of time. It is very difficult for an operator to program these changes “on the fly” and have each of these changes over simultaneously over time. The common programming platforms, however, allow scenarios to be programmed in a way that by clicking one state such as “improvement” or “fluid administration,” all of these changes begin at once and occur over a specified time (Fig. 15.4).
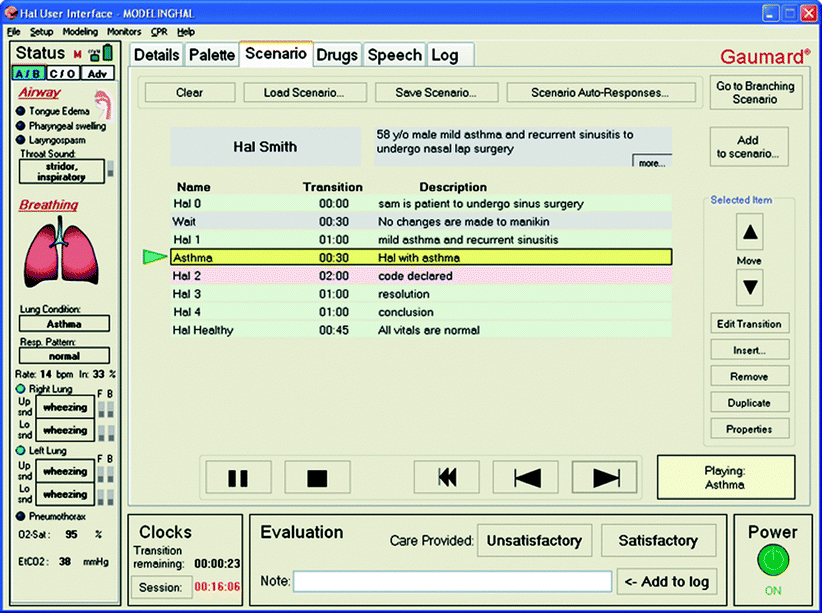

Fig. 15.4
Scenario palette of the HAL® user interface (Photo courtesy of Gaumard Scientific 2012. All rights reserved)
Operator-driven simulators can be programmed by adjusting vital signs, simulator voice/sounds (i.e., vomiting, grunting, crying), and physical exam findings. The programmer must have a working knowledge of expected changes caused by various treatments. Either too great, small, fast, or slow decreases the believability of the scenario. If the person programming the scenario has limited clinical experience, it is important that they work with someone more familiar with clinical manifestations to carefully adjust magnitudes and speed of treatment effects by someone with clinical experience.
Trending is a helpful programming feature that prevents sudden unbelievable vital sign changes. Trending changes can be programmed to occur over seconds to minutes. Examples of effective changes done through the use of trends are:
1.
Administration of oxygen – Increase saturations 5–10% over 30–60 s depending on scenario pathophysiology.
2.
Administration of intravenous fluids – Decrease heart rate by 5–10 beats per minute over 30–60 s and increase blood pressure by 5–10 mmHg over 30–60 s.
3.
Administration of neuromuscular blocking agent – Decrease respiratory rate to zero over 5–30 s depending on particular agent administered.
4.
Administration of dopamine – Increase blood pressure by 5–10 mmHg and increase heart rate 5–10 beats per minute over 30–60 s.
5.
Seizure – Increase heart rate by 25–50 beats per minute, increase blood pressure 20–40 mmHg, over 5–10 s, and increase end-tidal carbon dioxide by 10–30 over 1–2 min.
6.
Administration of anticonvulsants to treat seizures – Reverse above changes over a similar time frame.
7.
Worsening – This trend can be programmed which the operator can activate when participants enter. This may be preferable to starting the physiologic changes when the scenario is opened. It can be hard to predict the exact entrance of participants.
8.
Shock – This can be an excellent example of a worsening change. Heart rate gradually increases, respiratory rate increases, end-tidal carbon dioxide decreases, and blood pressure decreases. Both systolic and diastolic pressure can decrease together or they can widen or narrow depending on the type of shock.
As these trends are created, they can be saved and used again for any other scenario. For example, many cases will have a component of hypoxia which is improved with oxygen. You can insert this trend into any of these cases.
Autonomous simulators are programmed by adjusting simulator physiology or by programming events that result in physiologic changes. For instance, if the operator wants to simulate shock, they may simply program the simulated patient to lose 10% of their blood volume over a desired time frame. Unlike operator-driven simulators that require the operator to actually change the vital signs, autonomous simulators will improve by infusing blood. The physiology of autonomous simulators is complex but sometimes needs to be adjusted slightly to make changes occur at a different rate or degree. Complex trending in autonomous simulators may be replaced by the use of scenarios that contain multiple physiologic changes in each state. In addition, if more than one physiologic change is programmed, these changes may interact and affect each other. It is crucial to test these combined physiology changes to ensure a reasonable degree of believability.
CAE Healthcare (formerly METI) has one programming platform that functions autonomously, HPS, and a newer programming platform that functions more as operator-driven, Müse® (Fig. 15.5). Laerdal and Gaumard (Fig. 15.3) platforms are mostly operator-driven but may still respond autonomously to some interventions. All platforms include the ability to log and time stamp most physiological, pharmacological, and event data. Some platforms also automatically log advanced values such as alveolar and blood gases, cardiac output, and hematocrit values. These logs can aid in the programming of scenarios and events based on past simulations that were, perhaps, initially performed “on the fly.”
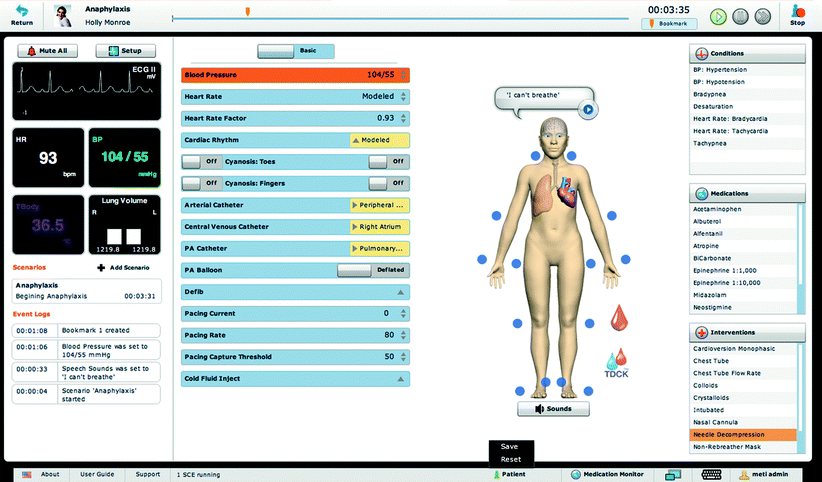

Fig. 15.5
CAE Healthcare’s Müse® user interface (Photo courtesy of CAE Healthcare ©2012 CAE Healthcare)
Nontechnical aspects of scenario programming are important as well. As shown in Fig. 15.1, supporting data such as mock laboratory results, appropriate radiographs improve the fidelity of the scenario. In addition, confederates such as parents, spouses, nurses, doctors, and consultants assist both the reality of the case as well as help move the scenario if learners are frustrated or misinterpret a finding which leads them to a completely different set of differential diagnoses and therapies. An example of this is the use of stridor in the Laerdal infant. This inspiratory sound can be misinterpreted as wheezing. If learning objectives include the differential diagnosis of stridor such as laryngotracheitis and foreign body, this will not be considered if the physical finding misinterpretation finding is not corrected. Confederates can gently redirect learners with statements such as “It sounded more like stridor than wheezing to me” or “I think the noise is occurring during inspiration and not expiration.” Their verbal cues as referenced in Fig. 15.1 are often crucial.
Feature Considerations
Respiratory
Most mannequins generate realistic breath sounds while the chest wall moves with respiration. Oxygen sensors are built in some models allowing for detection of changes in inspired oxygen concentration and, according to physiologic models, change the saturation accordingly. In addition, more advanced mannequin modeling considers patient-specific factors such as weight, functional residual capacity, cardiac output, shunt, and dead space. The rate of saturation and desaturation may be adjusted in some mannequins giving the instructor more control over timing of the scenario. Some models include a gas analyzer and may detect anesthetic gases (Fig. 15.6).
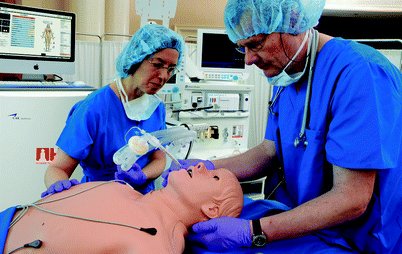

Fig. 15.6
METI HPS® Human Patient Simulator has an optional anesthesia delivery system (Photo courtesy of CAE Healthcare ©2012 CAE Healthcare)
Some mannequins may be attached to an external carbon dioxide source to simulate end-tidal carbon dioxide. Mannequins capable of exhaling carbon dioxide may do so in one of two ways. Some mannequins release an unprecise amount of carbon dioxide intended solely for qualitative assessment (e.g., to produce a positive response with a colorimetric carbon dioxide detector). Other mannequins produce model-driven condition-specific carbon dioxide waveforms capable of generating a capnogram.
Use of computer-controlled mannequins in the intensive care setting is limited by the capacity to respond physiologically to complex changes in mechanical ventilator modes and settings (PEEP, I-E ratios, pressure control, etc.). More recently simulators have been introduced with dependable dynamic airway and lung compliance, variable respiratory rates, and I-E ratios to accommodate mechanical ventilatory support. Most mannequins do, however, interface well with high-frequency oscillatory ventilation.
Cardiovascular
Pulse volume and strength is typically palpable at multiple anatomic locations and may dissipate with decreasing blood pressure. A range of heart sounds is present and may be auscultated using a standard stethoscope (Fig. 15.7). Most high-fidelity mannequins include a library of rhythms, and some are capable of generating a real electrocardiogram signal when connected to real equipment (Fig. 15.8). Many valvular heart diseases may be simulated and autonomous mannequins demonstrate hemodynamic behavior that reflects valvular lesions automatically. Learners sometimes find it difficult to hear or interpret mannequin heart sounds accurately with the notable exception of Harvey® the cardiopulmonary patient simulator designed for this purpose. Some mannequins simulate realistic jugular venous distension based on cardiac status. Almost all models are capable of full chest compressions and some provide feedback on effectiveness (Fig. 15.9).
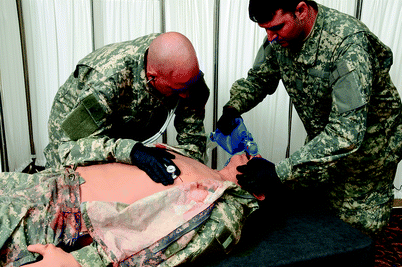


Fig. 15.7
Heart sounds being auscultated on METI iStan® (Photo courtesy of CAE Healthcare ©2012 CAE Healthcare)

Fig. 15.8
HAL® S3201 connected to real 12-lead ECG (Photo courtesy of Gaumard Scientific 2012. All rights reserved)

Fig. 15.9
Chest compressions performed on METIman® (Photo courtesy of CAE Healthcare ©2012 CAE Healthcare)
Airway
Most mannequin simulators allow for realistic bag-valve-mask intervention as well as endotracheal and nasotracheal intubation. Depth and position of endotracheal tube placement are sensed and respond appropriately in some models. Gastric distension typically results if a tube is placed in the esophagus. Operators may manipulate airway conditions to facilitate cannot ventilate and/or cannot intubate scenarios. This may be achieved through glottic and/or tongue swelling, trismus, laryngospasm, or bronchial occlusion. Cricothyrotomy is possible through replaceable neck skins. Some models are capable of oral secretions to add even more realism to airway manipulation.
In most situations, a dedicated airway trainer is superior to full-body mannequin simulators in terms of airway realism and manipulation. Even so, most model-based mannequins have an airway capable of basic airway interventions including placement of endotracheal tubes and laryngeal mask airways, mask ventilation, jet ventilation, and cricothyrotomy. In most cases, advanced airway techniques such as double-lumen tracheal tube placement and retrograde intubation are also possible. Mannequins with realistic tracheal and bronchial anatomy allow for direct laryngobronchoscopy and even practice of foreign body removal.
Neurologic
Many mannequin simulators have eyes that open, blink, and close depending on the state of consciousness (Fig. 15.10). Some mannequins feature pupils that change automatically in response to light or programmed states, while other mannequins have eyes that may be manually rotated to select a pupil size. Many full-scale mannequins also include embedded speakers that allow an operator to speak as the patient making the system a virtual standardized patient. The capacity to seize is included in some models though it is generally limited to movement of the head in adults and children or torso in infants. Degree of drug-induced paralysis may be monitored in at least one mannequin model by use of a peripheral nerve stimulator that simulates adductor pollicis muscle twitches. Some infant mannequins allow for assessment of the anterior fontanelle which may be bulging in when intracranial pressure in elevated.
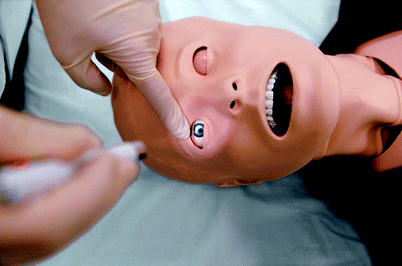

Fig. 15.10
SimMan® 3G eye signs (Photo courtesy of Laerdal Medical. All rights reserved)
Extremities
Generally all mannequin simulators have some degree of articulation though some are more limited than others. Some mannequins are capable of circumoral and/or peripheral cyanosis (Fig. 15.11). Various add-on kits are available to facilitate trauma conditions such as amputations (Fig. 15.12).


Fig. 15.11
Newborn HAL® S3010 includes cyanosis as a standard feature (Photo courtesy of Gaumard® Scientific 2012. All rights reserved)

Fig. 15.12
CAE Caesar™ trauma patient simulator is built with modular limbs that may be moulaged for amputation (Photo courtesy of CAE Healthcare ©2012 CAE Healthcare)
Pharmacologic Intervention
Select mannequins include drug recognition systems that identify drug, concentrations, and dosages administered via a bar-coded syringe filled with sterile water (Fig. 15.13) or radiofrequency identification tags (Fig. 15.14). These mannequins have varying degrees of pharmacologic modeling to autonomously initiate a realistic response. Some mannequins may be fitted with anesthesia delivery kits to allow for simulation of various anesthetic gas administrations.

Fig. 15.13
METI HPS® Human Patient Simulator drug recognition system uses barcode technology to accurately identify drug administered, concentration, and dose (Photo courtesy of CAE Healthcare ©2012 CAE Healthcare)

Fig. 15.14
SimMan® 3G advanced drug recognition system uses radiofrequency identification tags (Photo courtesy of Laerdal Medical. All rights reserved)
Procedural Intervention
Mannequins may be used for training invasive procedures such as chest tube insertion, needle thoracotomy, pericardiocentesis, diagnostic peritoneal lavage, urinary catheter placement, surgical cricothyroidotomy, and various other interventions. Intravenous catheter placement and arterial sampling are available on some models and may result in blood “flashback” to assist the learner in verifying placement. Mannequin skin and internal tubing, however, are rarely durable enough to make this a practical procedure during routine simulations. Most pediatric mannequins are capable of intraosseous access and infusion as well.
Interface with Existing Equipment
Vital signs are typically displayed on an accompanying monitor. Some mannequin simulators have the ability to interface with standard patient monitoring equipment, while other simulators require a monitor to be attached to the control computer that generates the clinical data that is displayed. Some manufacturers also include the ability to display clinical images such as chest radiographs (Fig. 15.15) or electrocardiograms.
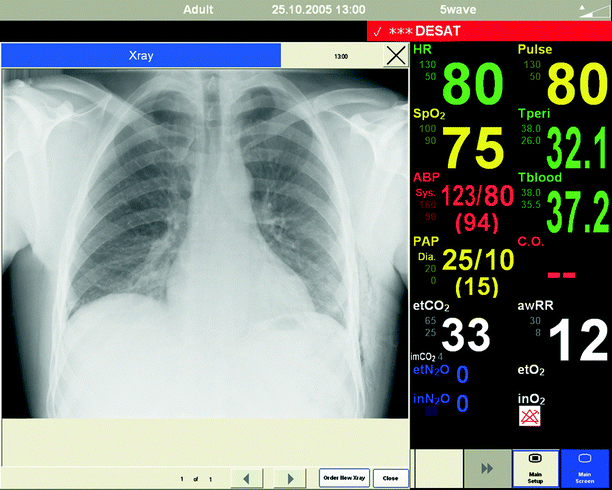

Fig. 15.15
Chest radiography displayed on SimMan patient monitor (Photo courtesy of Laerdal Medical. All rights reserved)
Portability
Mannequins often are used in a fixed location though more recently developed mannequins are more portable. Institutions utilizing in situ simulations value mannequins that are easily transported. In addition, simulators are sometimes transported outside the facility for training purposes. Mannequin portability has greatly increased in recent years due to availability of tetherless mannequins, rechargeable batteries, and reduced size of associated equipment including cabling, hoses, and compressors necessary to operate the mannequin. Many pediatric simulators and associated equipment can be contained in a portable crib or isolette.
Cost
Although not a “feature” per se, another significant point of comparison for mannequin-based simulators are the costs. In general, cost will rise with the degree of technicality and advanced features. The initial purchase price should be considered as well as costs associated with service contract, upgrades, associated software, and hardware requirement. It is not uncommon for new features to become available that may require an additional investment. Because simulators vary in their ease of operability, another cost consideration is the degree to which varying levels of operators may be trained to use the equipment.
Regardless of features, it should be noted that mannequin simulators today are fairly unreliable compared to comparatively priced devices used on a daily basis. The degree of unreliability seems to increase with the complexity of the mannequin. Busy centers realize the importance of having an experienced simulationist that can quickly address these sudden problems. It is generally advisable to maintain warranty coverage in order to address these expected malfunctions and required repairs.
Examples of Mannequin Simulators
At the writing of this chapter, there are three primary companies that produce programmable mannequin-based simulators in North America: Laerdal (Stavanger, Norway), CAE Healthcare (Sarasota, Florida), and Gaumard (Miami, Florida). All three companies manufacture adult and pediatric simulators.
Adult Mannequin Simulators
Though features differ between models and companies, mannequins with similar price points are generally comparable (Table 15.1; Figs. 15.16, 15.17, 15.18, and 15.19). Although prescribing every mannequin in detail is beyond the scope of this chapter, a few adult mannequins with distinguishing characteristics are described below. For completeness the reader is referred to Table 15.1 for side by side comparisons of equipment.
Table 15.1
Comparison of mannequins
Adult mannequin simulator comparison | |||||||||
|---|---|---|---|---|---|---|---|---|---|
HPS (CAE) | iStan (CAE) | METIman (CAE) | ECS (CAE) | SimMan Essential (Laerdal) | SimMan 3G (Laerdal) | HAL S3000 (Gaumard) | Hal S3201 (Gaumard) | Susie 2000 (Gaumard) | |
General | |||||||||
Autonomous | Yes | Yes | Yes | Yes | No | No | No | No | No |
Tetherless | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
Real monitors | Yes | No | No | No | No | No | No | Yes | Yes |
Airway/respiratory | |||||||||
Trismus | No | Yes | No | No | Yes | Yes | No | No | No |
Articulating mandible | No | Yes | No | No | Yes | Yes | No | Yes | No |
Breakaway teeth | Yes | Yes | Yes | Yes | No | No | No | No | No |
Nasal intubation | Yes | Yes | Yesa | Yes | Yes | Yes | Yes | Yes | No |
Bronchial occlusion | Yes | Yes | Yes | Yes | No | Yes | No | Yes | No |
Laryngospasm | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
Airway occlusion | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
Airway resistance | Yes | Yes | Yesa | Yes | Yes | Yes | No | Yes | No |
Oral secretions | Yes | No | Yesa | No | No | Yes | No | No | No |
Cardiovascular | |||||||||
CO2 detection | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No |
JVD | No | Yes | No | No | No | No | No | No | No |
Cyanosis | No | Yes | No | No | No | Yes | Yes | Yes | Yes |
Capillary refill | No | Yes | No | No | No | No | No | No | No |
Pulse sites | Yes | 14
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |||||||





