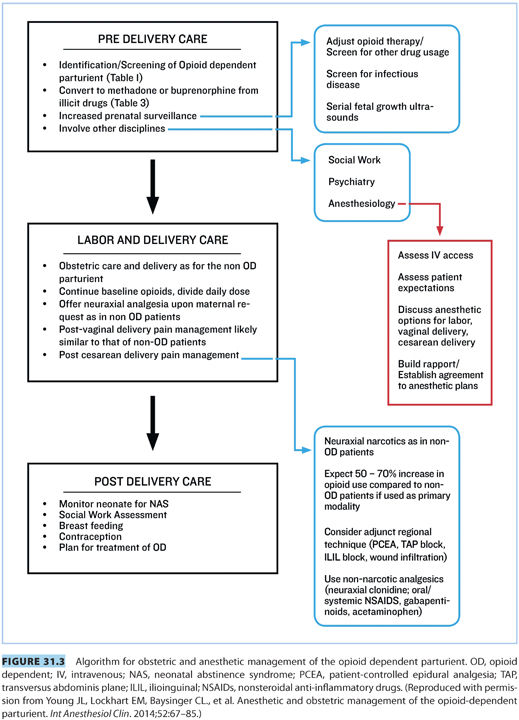
II.Obstetric management (see Fig. 31.3)
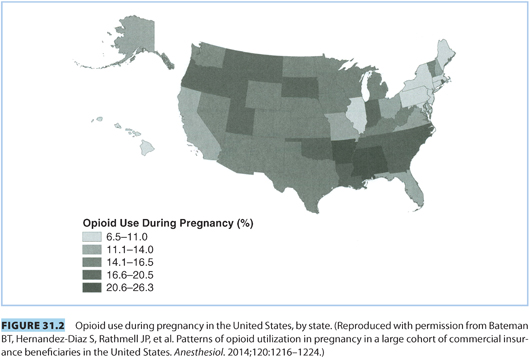
A. Risks of opioid dependence in pregnancy
1. Opioid dependence in pregnancy is associated with poor maternal and fetal outcomes including miscarriage, preterm labor, preterm delivery, premature rupture of membranes, intrauterine growth restriction, and NAS.
2. Withdrawal symptoms during pregnancy can be dangerous for the fetus because it causes maternal tachycardia, hypertension, decreased placental perfusion, and uterine contractions.1
3. Women in the cycle of intoxication and withdrawal are at higher risk for intrauterine growth restriction, insufficient nutrition, or exposure to illicit opioids such as heroin.
4. Rates of infectious disease, such as hepatitis C, are higher in this population.2,3
5. Due to a combination of psychosocial risk factors, this patient population is at risk for delayed or inadequate prenatal care.
6. Poor fetal outcomes and increased risk of neonatal abstinence can be attributed to the concurrent use of other substances including tobacco, alcohol, benzodiazepines, and other illicit drugs.4–6
7. Concurrent psychiatric disorders are also common in this population, with one 2010 study reporting up to 65% of these patients have symptoms of mental illness.7
8. Opioids are not thought to be teratogenic, particularly with short-term use.
a. Most large retrospective studies have shown no association between opioid exposure and congenital anomalies8; however, one retrospective study suggests an association between codeine exposure in the first trimester and cleft palate, cardiac defects, and pyloric stenosis.9
b. More recently, the 2011 National Birth Defects Prevention Study showed an association between cardiac, spinal, and abdominal wall defects with opioid exposure in the first trimester, but these results have not been replicated.10
CLINICAL PEARL Opioid dependency during pregnancy is associated with poorer obstetric and neonatal outcomes due to increased rates of viral and nonviral infectious disease, opioid withdrawal, use of illicit substances, and less prenatal care. Opioid dependency is aggravated in many cases by concomitant maternal psychiatric illness.
B.Identification. Identification of the OD woman early in pregnancy is important for early treatment, education, and intervention.
1. The American College of Obstetricians and Gynecologists (ACOG) suggests that all pregnant women be screened for substance abuse with a validated screening tool such as 4P’s Plus or the Car, Relax, Alone, Forget, Friends, Trouble (CRAFFT) screening questionnaires.11–14
2. Universal urine drug testing is not recommended during pregnancy because of informed consent issues and testing limitations.
3. Urine drug testing can be a valuable tool to measure adherence to a treatment plan, if done with transparency and consent.15
4. Prior to ordering toxicology screening, the provider should be familiar with the scope and limitations of the available tests, including the metabolites of commonly abused drugs and medications that cause false-positive results.
5. Once identified as OD, a patient should be educated about the risks of opioid dependence in pregnancy and referred to an addiction treatment program.
CLINICAL PEARL All pregnant women should be screened for opioid use using validated screening tools; the use of screening urinalysis is not recommended.
C.Treatment. Maintenance therapy with methadone or buprenorphine is the standard of care for treatment of opioid addiction in pregnancy.
1. Methadone maintenance has been the standard of care for the treatment of opioid addiction in pregnancy for decades.
a. Women on methadone maintenance are more likely to attend prenatal visits, have improved obstetrical outcomes, and are less likely to have children placed in foster care.15
b. There is evidence that methadone maintenance reduces the risk of NAS 50% to 75%.16
c. Disadvantages of methadone maintenance therapy include cost, requirements of daily visits to a licensed treatment facility, and social stigma.
2. Buprenorphine is a μ-opioid receptor agonist which is also being used for treatment of opioid dependence during pregnancy.
a. In the Maternal Opioid Treatment: Human Experimental Research (MOTHER) trial, women treated with buprenorphine were shown to have comparable outcomes in the treatment of opioid dependence to those women receiving methadone.17
b. The advantages to buprenorphine are that it is an office-based treatment and is often covered by insurance.
c. The symptoms of NAS in babies exposed to buprenorphine may be less severe.17
d. Due to its high affinity for the μ-opioid receptor, buprenorphine cannot be administered to someone who has recently used other opioids because it might precipitate withdrawal symptoms.18
3. Detoxification is generally not recommended during pregnancy.
a. The risk of relapse during pregnancy is high following detoxification. One retrospective study showed that inpatient detoxification was initially successful in 53 of 95 addicted women, but 45% of those women relapsed.15
b. If not done carefully, detoxification can trigger withdrawal symptoms leading to preterm labor or premature delivery.18,19
c. The average length of inpatient stay for the women who are successfully detoxified is about 25 days.20
CLINICAL PEARL Opioid-addicted women should receive long-acting opioid maintenance therapy because it is associated with better obstetric and neonatal outcomes; detoxification from opioid use should be discouraged.
D.Chronic pain in pregnancy. Chronic pain in pregnancy requiring opioid therapy may be managed using a patient’s current regimen as long as there is no sign of abuse or addiction.
1. There is limited data but a few studies suggest that NAS is less common in this population with an incidence of 11% to 38%.17,21
2. Treatment plans must be individualized with consideration of maternal quality of life, fetal risks, and patient goals.
3. Opioid therapy in these instances should not be stopped abruptly but instead tapered if detoxification is appropriate.
E.Peripartum obstetric management. Peripartum obstetric management itself in OD women does not differ appreciably from that of non-OD women.
1. Serial ultrasounds are recommended after 24 weeks of gestation to monitor fetal growth.22
2. There is no evidence to support additional antenatal testing without other pregnancy-related risk factors.
3. The frequency of routine visits may be increased in order to monitor adherence to addiction treatment.
4. Delivery plans do not need to be altered if the patient is stable in treatment.
CLINICAL PEARL Obstetric management of OD women should be the same as in non-OD women.
III.Neonatal abstinence syndrome
A.Definition and incidence
1. NAS appears after the sudden discontinuation of transplacental opioids at birth and represents neonatal dependence.
2. Symptoms are noted in approximately 50% to 80% of opioid-exposed neonates after birth.23–25
3. If left untreated, NAS can result in seizures and death.25,26
B.Symptoms
1. Symptoms reflect central nervous system, gastrointestinal, and autonomic nervous system dysfunction and can indicate the severity of withdrawal and need for treatment.
2. Symptoms include high-pitched cry, feeding difficulties, tremors and hypertonia, sweating, fever, and tachypnea.
C.Diagnosis
1. The American Academy of Pediatrics (AAP) recommends the use of an assessment tool such as the Finnegan Neonatal Abstinence Scoring Tool (FNAST).27
2. Maternal history and drug testing and fetal urine and meconium testing can also be useful.
3. Decisions regarding treatment are based on a cumulative threshold score.27 For example, two or more consecutive FNAST scores of 8 or 9 is a common indicator for intervention.28
4. Breastfeeding lowers the incidence of NAS and leads to shorter duration of treatment in infants with NAS.29
CLINICAL PEARL Breastfeeding is encouraged in women who are opioid dependent because it reduces the incidence of NAS.
D.Risk factors
1. The dose of methadone does not influence the rate of NAS diagnosis but does affect its severity and the need for treatment.30–32
2. Preterm birth does not influence the odds of the infant receiving treatment.
3. Timing and mode of delivery as well as timing of the last maternal methadone dose are significant risk factors for the development of NAS requiring treatment.24
4. The need for pharmacologic therapy is affected by genetics, other drug exposures, gestational age, breastfeeding, and maternal rooming-in.28
5. Maternal buprenorphine use may reduce the incidence and severity of NAS when compared to other opioids.17
E.Treatment of neonatal abstinence syndrome
1. Pharmacologic intervention is required for 50% to 70% of infants with NAS who demonstrate escalating symptoms.
2. Once symptoms resolve, treatment can be weaned over a period of days to weeks.33
3. Methadone and morphine are most commonly used to treat NAS.
4. The efficacy of clonidine has not been proven, but can be used as an adjuvant to other therapies.27
5. Barbiturates and benzodiazepines have been used but are not currently recommended as primary agents.27
6. The use of naloxone is contraindicated because it may induce neonatal seizures.27
CLINICAL PEARL NAS probably affects nearly all of the newborn offspring of OD mothers. Increasing severity of symptoms on repeat assessment tests (e.g., FNAST) necessitates treatment with a long-acting opioid.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








