Chapter 87
Management of Postoperative and Other Acute Pain 
Undermedicating Postoperative Pain
Finally, there is a lag time in the delivery of prn opioids between when the patient requests pain relief and when he or she actually receives that relief. Typically, a nurse must first respond to a patient’s call bell and locate, prepare, and administer the opioid, after which the patient must await the therapeutic effect of the opioid. This lag time becomes even more problematic when, as is typical, the patient does not request relief until the pain is overwhelming. Pro re nata administration is more effective if frequent small doses of intravenous (IV) analgesic are immediately available. Indeed, this is the basis of patient-controlled analgesia (PCA).
Assessment of Pain
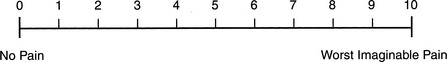
In alert, conscious patients, pain can be objectively assessed using a visual analog pain scale (VAPS) (Figure 87.1). This easy-to-understand system helps to avoid misinterpretation. Patients generally do not choose values on either extreme, and a VAPS score of 3 (or less) is considered to represent acceptable pain control in an ICU setting. There is usually always some patient discomfort, even if unrelated to the surgery (e.g., intravenous catheters, nasogastric tube, bed-bound status, tape, and so on). The choice of values on one extreme or the other of the VAPS generally implies a stoic or emotive personality.
Rationale for Using Preemptive Analgesia
Effective preoperative analgesia often decreases postoperative pain in a manner that exceeds expectations based solely on the pharmacodynamics and pharmacokinetics of the drugs administered. Studies support a concept of postinjury peripheral and spinal nerve hypersensitization and spinally mediated neuroplasticity following pain perception (nociception). Neuroplasticity implies that the central nervous system and the dorsal horn cells adapt in response to noxious stimulation. For example, repetitive stimulation of small pain fibers produces a progressive increase in action potential discharge (wind-up) and a prolonged increase in the excitability of spinal neurons with which they synapse. Central sensitization predisposes dorsal horn nociceptive neurons to respond to the input of normally innocuous Ab afferent fibers. Spinal sensitization seems dependent upon N-methyl-d-aspartate acid (NMDA) receptor stimulation and may be prevented by N-methyl-d-aspartate acid receptor antagonists administered before or after the peripheral injury. Small clinical studies have elucidated improved pain scores, decreased regions of perceived hyperalgesia, and decreased opioid requirements up to 6 months postoperatively following rectal carcinoma resection through the use of subanesthetic doses of ketamine via continuous infusion during anesthesia for surgery.
Methods of Controlling Postoperative Pain
Patient-Controlled Analgesia
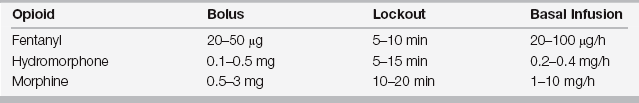
The demand dose is immediately responsive to the patient’s perceived pain and permits titration of analgesics to the minimal effective analgesic concentration, thus reducing periods of excessive pain or sedation. Patients act as their own “nocistat” with frequent small prn (demand) boluses. Analgesic administration is reduced to a simple feedback loop: The patient’s request for analgesia is rapidly honored by the PCA pump, which minimizes the “lag time.” In effect, PCA optimizes the traditional prn opioid cycle. The frequency of administration of the demand dose is determined by a preset interval (lockout time), which is predicated on the pharmacokinetics of the medication being used (Table 87.1). ![]()
In theory, the basal continuous infusion prevents significant decreases in the serum level of opioid while patients sleep, so they can avoid awakening with severe pain. The use of a basal infusion, however, has been demonstrated to increase opioid use without improving overall patient satisfaction or VAPS score. Still, respiratory depression has only rarely been reported with reasonable doses of PCA, and ICU patients on positive-pressure ventilators are generally safe with continuous basal infusions. Respiratory depression has been reported following postoperative hemorrhage because of a reduction in the volume of distribution that resulted in relatively high opioid concentrations. One report of meperidine overdose was attributable to a “runaway” malfunction of the PCA pump. However, meperidine is not recommended for PCA because of the potential for accumulation of normeperidine, a central nervous system (CNS) excitatory metabolite with a long half-life (Chapter 17). Normeperidine toxicity is more likely in patients with renal dysfunction. If a basal infusion is used with PCA, it must be appreciated that sleep and coadministered sedatives (Chapter 4) are synergistic in their respiratory depressant effects.
Dexmedetomidine
Dexmedetomidine is very useful for ICU procedures because of its sedative and analgesic properties. In contrast to the gamma-aminobutyric acid (GABA) agonists commonly employed for sedation in a postoperative ICU stay (benzodiazepines), dexmedetomidine yields sedation with easy awakening and a synergy with opioid analgesics (sparing effect) through alpha 2 CNS receptor agonist effects. In this regard, dexmedetomidine is about eight times as potent, has more alpha 2 specific effects, and has a shorter half-life when compared to clonidine, another commonly used alpha 2 adrenergic receptor agonist. A reduced incidence of patient delirium and agitation has been reported as compared to more traditional equivalent dosages of combined midazolam/fentanyl sedation and analgesia or with propofol sedation. Moreover, the respiratory depression associated with opioid use can be eliminated, resulting in shorter ICU stays and more rapid endotracheal extubations. Dexmedetomidine has no significant depression of minute ventilation or respiratory response to elevated PaCO2 or decreased PaO2. In fact, dexmedetomidine has been used as a successful bridge for patients who suffer emergence delirium from more conventional benzodiazepine/opioids or volatile agents. However, it has not been useful for treatment of acute agitated delirium (Chapter 37).
Dexmedetomidine dosing is often initiated with the administration of an IV bolus dose, which is associated with a predictable bradycardia. If this is undesirable, dexmedetomidine may be administered as a continuous IV infusion without a loading dose, wherein the peak effect is achieved after 2 hours of continuous infusion. Other medications can be used to supplement the sedative or analgesic properties of dexmedetomidine, and a dosing paradigm is shown in (Figure 87.2). Stable hemodynamics are commonly maintained when this regimen is utilized. Dexmedetomidine may be relatively contraindicated in patients with free flap reconstructive surgery or after cerebrovascular interventions, as alpha 2 agonists may precipitate a local vasoconstriction (in denervated arteries). High-grade atrioventricular (AV) block has also been a reported side effect in patients and may require pacing.
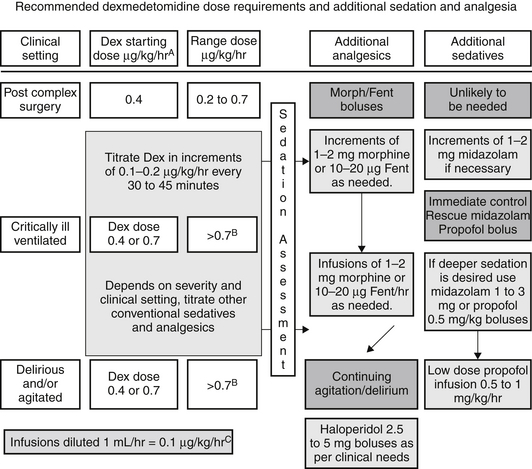
Figure 87.2 Schematic flow diagram of a protocol for dosing of dexmedetomidine with adjunctive agents for intensive care sedation. Dex, dexmedetomidine; Fent, fentanyl.
AInfusions should always start at 0.4 μg/kg/hr for one hour and be increased thereafter as required. Assessment of sedation and pain scales should be performed as part of ongoing evaluation at least every 4 hours.
BThe maximum dose above 1 μg/kg/hr is not identified or approved. However, according to published literature, it is reasonable to use a dose up to 1.5 μg/kg/hr.
CThis can be achieved by adjusting the volume of 5% DW or N/S added to 200 μg (full vials of dexmedetomidine) so that every milliliter contains 0.1 μg/kg of dexmedetomidine. This will also avoid discarding any dexmedetomidine, thereby avoiding any wastage. (Adapted from Shehabi Y, Both JA, Ernest D et al: Clinical application: the use of dexmedetomidine in intensive care sedation. Crit Care Shock 13:40-50, 2010.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree