Management of pain in the critically ill patient
Mario De Pinto
W. Thomas Edwards
Pain is a major problem for patients hospitalized in intensive care units (ICUs) [1, 2, 3, 4, 5]. In a study by Whipple et al., [1] assessing pain treatment in 17 patients with multiple trauma wounds, whereas 95% of house staff and 81% of nurses reported adequate analgesia, 74% of patients rated their pain as either moderate or severe.
Ineffectiveness of opioid analgesia in the intensive care setting is related more to the manner in which the medications are used than to the properties of the analgesics themselves [6]. Pain is frequently treated inappropriately because of fears of depressing spontaneous ventilation [7], inducing opioid dependence, and precipitating cardiovascular instability. Moreover, the methods for assessing pain, the techniques for aggressively treating it, and the beneficial results of effective pain management are often poorly understood by many clinicians [8, 9, 10, 11 12, 13, 14 and 15].
During the past 2 decades, researchers have discovered that the persistence of severe, inadequately treated pain can lead to anatomic and physiologic changes in the nervous system [16]. The ability of neural tissue to change in response to repeated incoming stimuli, a property known as neuroplasticity, can lead to the development of chronic, disabling pain when acute pain is poorly treated. Furthermore, the stress response after surgery and trauma, which includes cytokine and acute-phase reactants release, elevated levels of catecholamines, cortisol, growth and adrenocorticotropic hormones, activation of the renin-angiotensin system, impaired coagulability, and an altered immune response, accounts for a large portion of the mortality in critically ill patients [17]. In several studies, inadequately treated acute pain has been shown to increase this response, resulting in higher morbidity and mortality [18,19]. Another important aspect to consider is the psychological effect that poorly treated acute pain has on patients when the most acute phase of their disease is over. Prolonged, severe anxiety stemming from uncontrolled pain after traumatic injury has been deemed to be a cause of posttraumatic stress disorder [20].
Revised standards for pain management evaluation and treatment have been developed by the Joint Commission of Healthcare Organizations and have become part of all hospital evaluations since 2001 [21]. One critical part of the process required by the Joint Commission of Healthcare Organizations is the assessment of the effectiveness of each intervention and modification of the treatment plan when an appropriate level of analgesia is not achieved [22]. Particular attention has also been dedicated to the need of staff education as well as to the development of a system in each institution to deliver pain treatments promptly; in particular, staff education has been shown to be effective in improving pain control [23, 24].
A correct and successful management of acute pain is based on the following fundamental principles:
Pain is a subjective phenomenon; always believe the patient when he or she says, “I have pain,” whether the observer thinks that the amount of pain reported is appropriate [25].
Establishing the correct cause of the patient’s pain before initiating treatment is of paramount importance.
Pharmacologic treatment should be based on establishing and maintaining adequate drug levels at active sites to achieve and maintain appropriate levels of analgesia and anxiolysis.
Continually reevaluate the therapy for its effectiveness and modify the approach, using different treatment modalities as needed.
Always consider the possibility of using regional analgesia techniques (neuraxial and peripheral nerve blockade) whenever possible. Regional analgesia, when used appropriately and effectively, helps reduce the total amount of opioid analgesics necessary to achieve adequate pain control and subsequently the development of potentially dangerous side effects.
Acute Pain Pathways
Acute pain begins with damage to the skin or deeper structures. Algogens, produced or released locally, stimulate peripheral nociceptors. The signal is propagated along the nociceptor fibers into the dorsal horn of the spinal cord, or, in the case of the cranial nerves, into the sensory nuclei. Modulation, either amplification or attenuation, of the signal can take place before the signal is transmitted into pain-specific areas of the brain and cerebral cortex. An increase in the excitability of spinal neurons and central sensitization will cause an increase in the response to painful stimuli [25]. Reflexes are generated all along the pain pathway, resulting in responses that can be beneficial (e.g., withdrawal from a noxious stimulus) or deleterious (e.g., sympathetic discharge causing neuroendocrine changes characteristic of the stress response) to the injured organism [26]. One of the reflex responses associated with trauma or surgery is
increased muscle tone and localized spasm, which increases oxygen consumption and lactic acid production. Increased sympathetic outflow causes tachycardia and increased stroke volume, cardiac work, and myocardial oxygen consumption. Supraspinal reflex responses to pain result in hypothalamic stimulation, increased sympathetic tone, increased release of catecholamines and other “stress” hormones, and decreased secretion of anabolic hormones. The postsurgical and trauma catabolic state can be worsened if the process continues.
increased muscle tone and localized spasm, which increases oxygen consumption and lactic acid production. Increased sympathetic outflow causes tachycardia and increased stroke volume, cardiac work, and myocardial oxygen consumption. Supraspinal reflex responses to pain result in hypothalamic stimulation, increased sympathetic tone, increased release of catecholamines and other “stress” hormones, and decreased secretion of anabolic hormones. The postsurgical and trauma catabolic state can be worsened if the process continues.
Each separate part of the pain pathway (periphery, spinal cord, brain, and cerebral cortex) can be influenced so that the nociceptive signal is diminished or eliminated.
Formulation of A Treatment Plan
When designing a treatment plan it is important to understand the characteristics of the pathologic process responsible for the patient’s pain.
The answer to the following questions is crucial in establishing the most effective therapeutic approach possible.
Where Does It Hurt?
Is the location of pain appropriate for the injury sustained, the pathologic process, or for the surgery performed? Unrecognized injuries or pathology responsible for acute pain may be present. Sometimes pain may have nothing to do with the current acute problem in patients with underlying problems with chronic pain.
How Much Does It Hurt?
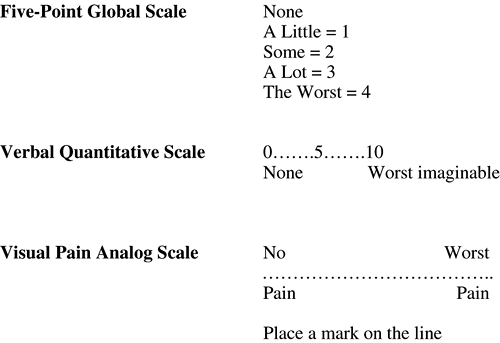
Each patient should be asked to try to quantify his or her pain using visual, verbal, or numeric analog scales [27, 28] (Fig. 23-1). With these measurements, each patient serves as his or her own control, and the response to treatment is best measured as change from the baseline value.
Pain in intubated and sedated patients can be assessed even if they are unable to communicate verbally. Various markers of sympathetic activity, such as restlessness, sweating, tachycardia, lacrimation, pupillary dilation, and hypertension can be graded as signs of pain intensity, although any one of these is not an adequate measure of pain intensity by itself [29].
What does it feel like?
Pain can be categorized as nociceptive and/or neuropathic. Nociceptive pain is transmitted through nonmyelinated C sensory fibers and small, myelinated A fibers [30] via the dorsal root ganglion and spinothalamic pathways in the spinal cord to the thalamus, periaqueductal grey, and other centers in the brain. Nociceptive pain encompasses somatic and visceral pain.
Somatic pain can be superficial, due to nociceptive input arising from skin, subcutaneous tissues, and mucous membranes; or it can be deep, arising from muscles, tendons, joints, or bones. It is usually described as sharp, pricking, well localized (superficial) or dull, aching, less well localized (deep).
Visceral pain is due to a disease process or abnormal function of an internal organ or its covering (parietal pleura, pericardium, peritoneum). It can be frequently associated with nausea, vomiting, sweating, and changes in heart rate and blood pressure.
Neuropathic pain is due to injury or dysfunction of the peripheral and/or central nervous system. Abnormal numbers and positioning of sodium channels in damaged neurons are responsible for the reduced response threshold and firing of action potentials even in the absence of a stimulus [31].
The N-methyl D-aspartate receptor is thought to be involved in the pathophysiology of neuropathic pain and other chronic pain states. In pathologic states, the N-methyl D-aspartate receptor is activated inappropriately, leading to a state of heightened excitability of pain pathways known as “wind-up” [32, 33 and 34].
Neuropathic pain is usually described as burning, tingling, and numbing and can be associated with allodynia (pain caused by normally innocuous stimulus) and hyperalgesia.
Breakthrough pain refers to an episodic surge in pain against a background of well-controlled pain. It can be an increase in the patient’s usual pain just before the next dose of regular analgesic.
Incident pain is a particular form of breakthrough pain, such as that brought on by movement or dressing changes.
Medical Management
ICU patients commonly have pain and physical discomfort from many factors such as preexisting diseases, acutely painful medical conditions, invasive procedures, or trauma [35]. Pain and discomfort can also be caused by monitoring and therapeutic devices (catheters, drains, endotracheal tubes), routine nursing care (e.g., airway suctioning, physical therapy, dressing changes) and prolonged immobility [24, 36]. Unrelieved pain may contribute to inadequate sleep, causing exhaustion and disorientation. Agitation in an ICU patient may also result from inadequate pain relief. Pain may also contribute to pulmonary dysfunction through localized guarding of muscles around the area of pain and a generalized muscle rigidity or spasm that restricts movement of the chest wall and diaphragm [37]. Effective analgesia tends to diminish pulmonary complications in postoperative patients [38].
Several studies have demonstrated that the combined use of analgesics and sedatives ameliorates the stress response in critically ill patients [19, 39, 40, 41, 42].
Nonpharmacologic Treatments
Nonpharmacologic interventions including attention to proper positioning of patients, stabilization of fractures, and elimination of irritating physical stimulation (e.g., proper positioning of ventilator tubing to avoid traction on the endotracheal tube) are important to maintain patient comfort. Studies have documented the successful use of transcutaneous electrical nerve stimulation in treating postoperative pain, pain associated with rib fractures, and burn pain [43, 44, 45]. One clinical investigation of burn patients shows that auricular acupuncture-like transcutaneous electrical nerve stimulation significantly reduces pain after dressing changes and wound debridement when compared to placebo [46].
Pharmacologic Treatments
Different classes of medications can be used to provide adequate analgesia in critically ill patients.
Nonsteroidal Antiinflammatory Drugs
Nonsteroidal antiinflammatory drugs (NSAIDs) interfere with the production of prostaglandins; they may diminish the inflammatory response to trauma and surgery and thereby decrease the nociceptive input; they also may augment analgesia when used in combination with opioids.
Ketorolac tromethamine (Toradol) has been shown to be effective in reducing opioid requirements after major surgery [49] without depressing the respiratory drive [50]; when used in combination with epidural blockade, it improves also chest and shoulder pain after thoracic surgery [51].
Ketorolac and other NSAIDs may cause nausea, peptic ulceration, and inhibition of platelet function [52, 53]. Severe bronchospasm may occur in patients with asthma, nasal polyposis, and allergy to aspirin [54, 55]; appropriate caution should be used when prescribing ketorolac in the unconscious patient. Ketorolac is contraindicated in the presence of acute or chronic renal failure as well as in the presence of hypovolemia and other conditions associated with decreased tissue perfusion; it should not be administered for more than 5 consecutive days and should always be used with appropriate monitoring of the renal function.
The role of the more selective COX-2 inhibitors in the critically ill patients remains unknown. Selective COX-2 inhibiting agents cause less gastrointestinal irritation with long-term use than traditional NSAIDs [56]. The slow onset of action of some agents may decrease their utility for acute pain management.
For patients in whom NSAIDs are contraindicated, acetaminophen may represent a good alternative. In combination with an opioid, acetaminophen produces a greater analgesic effect than higher doses of the opioid alone [57]. Care must be taken to avoid excessive and potentially hepatotoxic doses, especially in patients with a significant history of alcohol intake or a poor nutritional status [58].
Opioids
Opioids remain the main pharmacologic means for the treatment of acute pain in the ICU patient (Table 23-1).
TABLE 23-1. Guidelines for Front-Loading Intravenous Analgesia | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
Desirable attributes of a useful opioid include rapid onset of action, ease of titration, lack of accumulation of the parent drug or its metabolites, and low cost. Of the commonly used opioids, fentanyl has the most rapid onset and shortest duration of action; it causes only minor hemodynamic changes and does not affect the inotropic state of the heart. Virtually all hemodynamic variables including cardiac output and systemic and pulmonary vascular resistance, are unchanged after large doses of fentanyl [59]. Because fentanyl
is lipid-soluble, the duration of action with small doses (50 to 100 μg) is short as a result of redistribution from the brain to other tissues. Larger cumulative doses, including the doses delivered via a continuous infusion, become dependent on elimination as opposed to redistribution with possible drug accumulation and prolonged effects [60].
is lipid-soluble, the duration of action with small doses (50 to 100 μg) is short as a result of redistribution from the brain to other tissues. Larger cumulative doses, including the doses delivered via a continuous infusion, become dependent on elimination as opposed to redistribution with possible drug accumulation and prolonged effects [60].
Morphine has a slower onset and longer duration of action; hypotension may result from vasodilation (secondary to release of histamine) and one of its active metabolites (morphine 6-glucuronide) may cause prolonged sedation in the presence of renal insufficiency.
Hydromorphone is a semisynthetic opioid that is 5- to 10-fold more potent than morphine, but with a similar duration of action; it has minimal hemodynamic effects and lacks a clinically significant active metabolite, and it causes minor to no histamine release [35, 60].
Methadone is a synthetic opioid agent with properties similar to morphine that can be given enterally and parenterally [60]. It has a much longer duration of action than morphine but a similar receptor-associated adverse effect profile, although it is less sedating if the appropriate dose is carefully titrated. Although methadone is not the drug of choice for an acutely ill patient whose hospital course is rapidly changing, it is a good alternative for the patient who has a long recovery ahead and an anticipated prolonged ventilatory wean. Often when the patient’s clinical conditions are stable, transitioning from fentanyl or morphine infusions to methadone intravenously (IV) or via a feeding tube can help simplify care regimens and decrease dependence on infusions [60].
Remifentanil has not been widely studied in ICU patients; it requires the use of a continuous infusion because of its very short duration of action. Furthermore, the rapid offset of analgesia with remifentanil may result in a greater incidence of pain at the time of the discontinuation of the infusion, especially when compared with other longer acting opioids [61]. The short duration of action could be beneficial in selected patients requiring interruptions of analgesia medications administration to allow neurologic examinations [62].
Opioids Side Effects
Adverse effects of opioid analgesics are common and may occur frequently, especially in ICU patients. These effects may aggravate the patient’s illness and prolong the clinical course [63].
Respiratory depression is a concern in spontaneously breathing patients or those receiving partial ventilatory support. Hypotension is usually the result of the combination of sympatholysis, vagally mediated bradycardia, and histamine release (when using morphine) [64, 65]; particular care should be taken in patients with hemodynamic instability [66]. Gastric retention and ileus are common in critically ill patients, and intestinal hypomotility is enhanced by opioids [67, 68]; routine prophylactic use of a stimulant laxative may minimize constipation. Opioids may increase intracranial pressure with traumatic brain injury, although the data are inconsistent and the clinical significance is unknown [69, 70 and 71]. Withdrawal phenomena after opioid use in critically ill adult patients have been reported in rare cases only [72]. Addiction in adult patients receiving opioids also seems to be unusual; in a large series of 11,882 patients treated with various opioids, only 4 were reported to have become addicted [73]. Methadone in high doses has also been associated with prolongation of the QTc interval in the electrocardiogram as well as the risk of development of torsade de pointes [74, 75 and 76].
Opioids Administration Techniques
Opioid an-algesics should be administered on a continuous or scheduled intermittent basis, with supplemental bolus doses as required [77]. When analgesics are administered only on an “as needed” basis, it is almost impossible to achieve a minimal effective analgesic plasma concentration with subsequent poor overall pain control [78].
When a continuous infusion is used, a protocol incorporating daily awakening from analgesia and sedation (sedation vacation) may allow more effective analgesic titration with a lower total dose of opioid; daily awakening may also be associated with a shorter duration of ventilation and ICU stay [79]. For patients in whom a long recovery and a prolonged ventilatory wean are anticipated, the use of a long-acting opioid (e.g., methadone) for background pain control in association with supplemental bolus doses of a short-acting opioid is indicated.
In patients who are not critically ill, patient-controlled analgesia (PCA), has been reported to result in stable drug concentrations, a good quality of analgesia, less sedation, less opioid consumption, and potentially fewer side effects, including respiratory depression [40, 80]. In addition, a basal rate or continuous infusion mode can be used for consistent analgesia during sleep. Patient selection is important when PCA is used, and particular attention should be paid to the patient’s cognition, hemodynamic reserve, and previous opioid exposure in order to achieve optimal treatment and minimize the possibility of adverse events. PCA devices can also be used for nurse-controlled analgesia.
Transdermal fentanyl represents a different treatment approach, which could be used in patients who are hemodynamically stable and with more chronic analgesic needs. The patch provides consistent drug delivery, but the extent of absorption varies depending on the permeability, temperature, perfusion, and thickness of the skin. Fentanyl patches are usually not a recommended modality for acute analgesia because of their 12- to 24-hour delay to peak effect [81], and similar lag time to complete offset once the patch is removed.
Ketamine
Ketamine is an intravenous anesthetic that has analgesic properties. It works by blocking the N-methyl D-aspartate receptor as well as activating the mu receptor [30, 60, 82]. Ketamine is usually metabolized in the liver, and its major metabolite is norketamine [83]. Norketamine is one-third to one-fifth as potent as ketamine as an anesthetic but it has been demonstrated to have analgesic properties [84, 85]. Ketamine also has a rapid onset and short duration of action [86].
Blood pressure and spontaneous respiration are maintained under ketamine anesthesia/analgesia [87]; also, ketamine has an important neuroprotective role and hinders the progression of cerebral ischemia [88], even though it has been reported that ketamine may increase intracranial pressure and cerebral blood flow in man [89]. Schwedler et al. [90] have shown that intracranial pressure, cerebral blood flow, and perfusion pressure are unchanged during ketamine anesthesia; in addition, when ketamine is administered, either in animals or humans, during controlled ventilation, only minimal if any increase in intracranial pressure can be observed [91, 92].
Subhypnotic doses of ketamine administered as infusions have been used for critically ill ICU patients who are very difficult to sedate with opioid and benzodiazepine infusions [93]. These low-dose ketamine infusions (less than 5 μg per kg per minute) do not seem to be associated with the usual adverse effects of ketamine such as hypertension, tachycardia, increased intracranial pressure, excessive secretions, and vivid dreams and hallucinations [94]. Because of its potential adverse effects, ketamine is not recommended for routine sedation and analgesia of the critically ill patient, but it can be useful for more difficult situations and/or when short surgical procedures with intense pain, such as placement of chest tubes, dressing changes, and/or wound debridement in burn patients are necessary [60, 94].
Other Drugs
Other medications such as anticonvulsants, antidepressants, sodium-channel blockers, and muscle relaxants (e.g., baclofen) have a minimal role in the management of acute pain in the critically ill patient, although they may become part of a strategy aimed at treating the patient’s pain problem beyond the time of his or her stay in the ICU.
Topical anesthetics can be used in association with short-acting opioids and/or ketamine for dressing changes in burn patients or in patients with large, open wounds (e.g., patients with necrotizing fasciitis). Particular attention has to be paid to the amount of medication used in order to avoid dangerous side effects/complications secondary to local anesthetic accumulation and toxicity.
Benzodiazepines, barbiturates, propofol, phenothiazines, butyrophenones are usually given in combination with opioids [95]. They are used for anxiolysis, sedation, and production of amnesia, but not for analgesia, because they have no analgesic properties.
Regional Analgesia Techniques
Recent studies suggest that advances in perioperative anesthesia and analgesia improve pain relief and patient satisfaction and can affect outcome in surgical and trauma patients. Neuraxial anesthesia and analgesia and peripheral nerve blockade have the potential to reduce or eliminate the physiologic stress response to surgery and trauma, decreasing the possibility of surgical complications and improving outcome [96, 97, 98 and 99].
Used alone or in combination with other treatment modalities, regional analgesia techniques are an invaluable tool to address pain-related problems in critically ill patients, but the indications for their use must be established correctly; postsurgical and trauma ICU patients are at risk for numerous complications and the use of an inappropriate regional analgesia technique can cause a deterioration of the patient’s clinical status, affecting a potentially favorable outcome.
The purpose of this section is to discuss risk and benefits of neuraxial and peripheral nerve blockade for the management of pain in the critically ill patient.
General Considerations
There are several general considerations [100] related to regional analgesia:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree