Study objective
There are limited data on the clinical presentations and management of dabigatran-associated major bleeding outside the clinical trial setting. The aim of this study is to describe clinical characteristics, interventions, and outcomes in patients with dabigatran-associated major bleeding who present to the emergency department (ED).
Methods
We performed a retrospective observational chart review study of dabigatran-treated patients with nonvalvular atrial fibrillation who presented with acute major bleeding to the ED. We searched electronic medical record databases cross-referencing medication lists and hemorrhage International Classification of Diseases, Ninth Revision ( ICD-9 ) and ICD-10 codes. We studied the resulting charts to yield confirmed nonvalvular atrial fibrillation in patients with an index event of major bleeding and at least 1 dose of dabigatran in the 5 preceding days.
Results
The electronic search yielded 284 cases, and we assessed 93 as ineligible, leaving 191 in the final cohort. Of these, 118 patients (62%) had gastrointestinal hemorrhage; 36 (19%) had intracranial hemorrhage, 8 (4%) of which were nontraumatic cases and 28 (15%) traumatic. Thirty-six (19%) of the index events were in “other” locations and 1 (0.5%) “unknown.” There were 12 deaths (6%): 8 from patients presenting with gastrointestinal bleeding events, 2 from intracranial hemorrhage (both nontraumatic), and 2 from other. Although RBC and plasma transfusions were common, only 11 patients (6%) received purified coagulation factors.
Conclusion
Despite rare use of reversal strategies, mortality was low and outcomes were favorable, similar to reported outcomes from clinical trials, in this sample of patients with major bleeding while receiving dabigatran.
Introduction
Background
The first decades of the 21st century have seen a significant change in the options for oral anticoagulation, with the advent of non–vitamin K oral anticoagulant agents for stroke prevention in nonvalvular atrial fibrillation, and prevention and treatment of venous thromboembolism. The non–vitamin K oral anticoagulant agents are rapidly replacing warfarin, the mainstay of oral anticoagulation for the past 50 years, prescribed 22 million times annually in the United States. Warfarin has long been a leading cause of drug-related adverse bleeding events. Multiple clinical trials have established that the non–vitamin K oral anticoagulant agents are at least as efficacious as warfarin for the prevention of stroke and systemic emboli in patients with nonvalvular atrial fibrillation and for the management of venous thromboembolism. Furthermore, the non–vitamin K oral anticoagulants have been associated with fewer major bleeding events than warfarin and tend to lead to less intracranial hemorrhage but more gastrointestinal bleeding.
What is already known on this topic
Dabigatran-associated bleeding is poorly described.
What question this study addressed
What were the major bleeding events, treatments, and outcomes in patients receiving dabigatran for nonvalvular atrial fibrillation before the era of specific antidotal therapy?
What this study adds to our knowledge
Of 191 eligible patients identified retrospectively from 5 emergency departments, 62% had gastrointestinal, 19% had intracranial, and 15% had trauma-related bleeding. The most common interventions were RBC and plasma transfusions, and 6% died (75% of those deaths from gastrointestinal bleeding).
How this is relevant to clinical practice
This knowledge will be useful in guiding development of protocols for the treatment of this condition.
Warfarin-related hemorrhages historically have been managed with vitamin K and fresh frozen plasma and more recently with prothrombin complex concentrates. Until recently, no specific reversal agents for the non–vitamin K oral anticoagulant agents have been available outside clinical trials. The first non–vitamin K oral anticoagulant agent approved for clinical use was dabigatran, a direct thrombin inhibitor. Dabigatran is most commonly prescribed for the risk reduction of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. As a consequence of their age and comorbidities, these patients are also the most likely to experience bleeding complications. For this reason, we focused on the population of nonvalvular atrial fibrillation patients who were receiving dabigatran to characterize a sample of anticoagulated major bleeding episodes.
Importance
The non–vitamin K oral anticoagulant agents, including dabigatran, were associated with fewer major bleeding events than warfarin in phase 3 clinical trials, and early reports after Food and Drug Administration approval suggested that dabigatran-induced bleeding had a more benign clinical course compared with that of warfarin. We sought to describe the characteristics, treatment, and outcomes of patients with major bleeding while receiving dabigatran in routine clinical practice.
Goals of This Investigation
Our goal was to assemble a multicenter cohort of nonvalvular atrial fibrillation patients with major bleeding while receiving dabigatran and characterize clinical presentations, treatment, and outcomes.
Materials and Methods
Study Design and Setting
We performed a study of nonvalvular atrial fibrillation patients who were receiving dabigatran, had an acute major bleeding event (index event), and presented to an emergency department (ED) at 5 sites in the United States. We identified subjects by electronic medical record searches cross-referencing medication lists and International Classification of Diseases, Ninth Revision ( ICD-9 ) and ICD-10 codes for hemorrhage. We then manually reviewed the charts for patients meeting the major bleeding criteria of the International Society for Thrombosis and Haemostasis. The 5 sites obtained individual institutional review board or independent ethics committee approval from their local institutional review board to conduct the study.
The period under review was from October 19, 2010 (when dabigatran was approved for the US market), to the date of institutional review board approval and subsequent electronic medical record query, which varied by site.
Because this study abstracted anonymous data devoid of patient identifiers, all institutional review boards granted patient written informed consent waivers. One or more experienced research assistants or coordinators at each site collected the data. They were aware of the general purpose of the study but not the details. Cases were reviewed by investigators at each site and entered into an electronic case report form and finally into a central database managed by an independent contract research organization. We did not formally assess interrater reliability by reabstracting a subset of the charts. The contract research organization’s medical monitor did query database entries for accuracy.
Selection of Participants
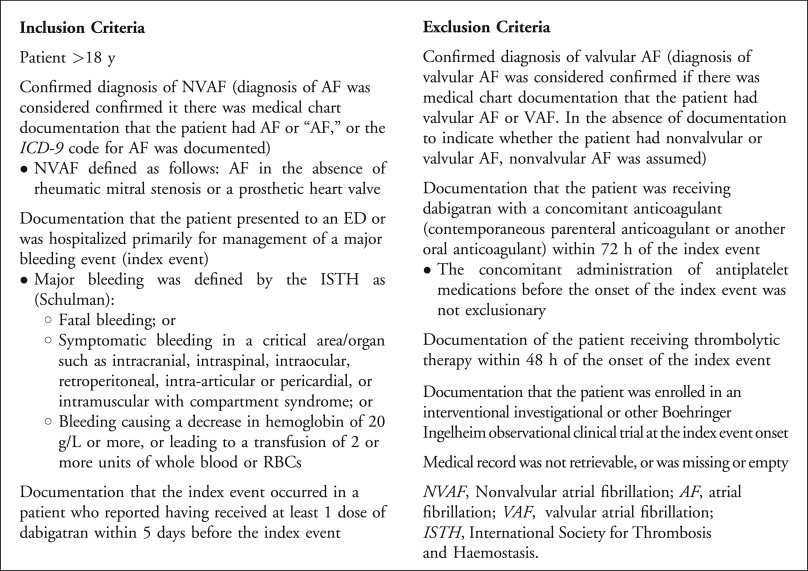
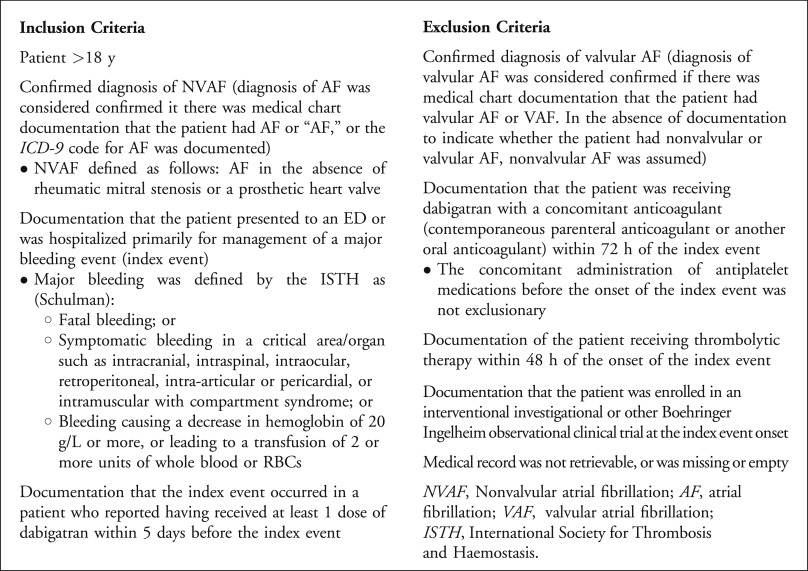
Patients were eligible for inclusion if they were aged 18 years or older, had a documented diagnosis of nonvalvular atrial fibrillation, had received a dose of dabigatran within 5 days of the index event, and presented to the hospital with a major bleeding episode (see Figure 1 for a complete list of inclusion and exclusion criteria). The International Society for Thrombosis and Haemostasis criteria defined major bleeding and included one of the following: fatal bleeding, bleeding into a major organ or critical area (eg, intracranial, retroperitoneal, pericardial, intraspinal, intra-articular, intraocular), a decrease in hemoglobin level of 20 g/L, or transfusion of at least 2 units of blood. Patients receiving additional oral or parenteral anticoagulants or thrombolytics were excluded.
Data Collection and Processing
We used a standardized electronic case report form to abstract information from the medical records. We collected specific details in regard to the timing and dosing of dabigatran, as well as the estimated time since nonvalvular atrial fibrillation diagnosis. We also noted the presence of renal failure, comorbidities, and concomitant medications, and the characteristics of the index bleeding event (type and location of bleeding).
Outcome Measures
The primary outcomes were the number of patients with an index event (ongoing, resolved, or deceased) at hospital discharge or release, the proportion of patients receiving different types of interventions to manage the index event (including intravenous fluids, blood and blood products, factor concentrates, and hemodialysis), frequencies of bleeding types, and anatomic locations of the index events at ED presentation. The status “ongoing” was not clinically defined and was left to the investigators’ discretion according to their review of the medical records. We also calculated total length of stay in the hospital and ICU.
Primary Data Analysis
We used descriptive statistics to summarize the study outcomes. We present binary data as the number and proportion of occurrence and continuous data as means and SDs or medians and interquartile ranges (IQR), as appropriate. We stratified outcomes according to baseline characteristics such as age and type of bleeding. We used SAS (version 9.2; SAS Institute, Inc, Cary, NC) to perform all statistical analyses.
Materials and Methods
Study Design and Setting
We performed a study of nonvalvular atrial fibrillation patients who were receiving dabigatran, had an acute major bleeding event (index event), and presented to an emergency department (ED) at 5 sites in the United States. We identified subjects by electronic medical record searches cross-referencing medication lists and International Classification of Diseases, Ninth Revision ( ICD-9 ) and ICD-10 codes for hemorrhage. We then manually reviewed the charts for patients meeting the major bleeding criteria of the International Society for Thrombosis and Haemostasis. The 5 sites obtained individual institutional review board or independent ethics committee approval from their local institutional review board to conduct the study.
The period under review was from October 19, 2010 (when dabigatran was approved for the US market), to the date of institutional review board approval and subsequent electronic medical record query, which varied by site.
Because this study abstracted anonymous data devoid of patient identifiers, all institutional review boards granted patient written informed consent waivers. One or more experienced research assistants or coordinators at each site collected the data. They were aware of the general purpose of the study but not the details. Cases were reviewed by investigators at each site and entered into an electronic case report form and finally into a central database managed by an independent contract research organization. We did not formally assess interrater reliability by reabstracting a subset of the charts. The contract research organization’s medical monitor did query database entries for accuracy.
Selection of Participants
Patients were eligible for inclusion if they were aged 18 years or older, had a documented diagnosis of nonvalvular atrial fibrillation, had received a dose of dabigatran within 5 days of the index event, and presented to the hospital with a major bleeding episode (see Figure 1 for a complete list of inclusion and exclusion criteria). The International Society for Thrombosis and Haemostasis criteria defined major bleeding and included one of the following: fatal bleeding, bleeding into a major organ or critical area (eg, intracranial, retroperitoneal, pericardial, intraspinal, intra-articular, intraocular), a decrease in hemoglobin level of 20 g/L, or transfusion of at least 2 units of blood. Patients receiving additional oral or parenteral anticoagulants or thrombolytics were excluded.
Data Collection and Processing
We used a standardized electronic case report form to abstract information from the medical records. We collected specific details in regard to the timing and dosing of dabigatran, as well as the estimated time since nonvalvular atrial fibrillation diagnosis. We also noted the presence of renal failure, comorbidities, and concomitant medications, and the characteristics of the index bleeding event (type and location of bleeding).
Outcome Measures
The primary outcomes were the number of patients with an index event (ongoing, resolved, or deceased) at hospital discharge or release, the proportion of patients receiving different types of interventions to manage the index event (including intravenous fluids, blood and blood products, factor concentrates, and hemodialysis), frequencies of bleeding types, and anatomic locations of the index events at ED presentation. The status “ongoing” was not clinically defined and was left to the investigators’ discretion according to their review of the medical records. We also calculated total length of stay in the hospital and ICU.
Primary Data Analysis
We used descriptive statistics to summarize the study outcomes. We present binary data as the number and proportion of occurrence and continuous data as means and SDs or medians and interquartile ranges (IQR), as appropriate. We stratified outcomes according to baseline characteristics such as age and type of bleeding. We used SAS (version 9.2; SAS Institute, Inc, Cary, NC) to perform all statistical analyses.
Results
Characteristics of Study Subjects
Of 284 patient charts we identified at the initial screening, 93 did not to meet the International Society for Thrombosis and Haemostasis major bleeding criteria, and we excluded them from the study, leaving 191 eligible charts. See Table 1 for enrollment by site.
| Site ∗ | Investigator | Screened | Ineligible | Included (%) |
|---|---|---|---|---|
| Maimonides Medical Center | Christian Fromm, MD | 26 | 5 | 21 (81) |
| Beth Israel Deaconess Medical Center | Michael Ganetsky, MD | 67 | 31 | 36 (54) |
| Seton Dell Medical School | Truman J. Milling, MD | 106 | 22 | 84 (79) |
| Stony Brook University Hospital | Adam Singer, MD | 61 | 28 | 33 (54) |
| Brigham and Women’s Hospital | Daniel J. Pallin, MD, MPH | 24 | 7 | 17 (71) |
| Totals | 284 | 93 | 191 (67) |
∗ Maimonides Medical Center is an urban, community, tertiary care, teaching hospital in Brooklyn, NY, and a Level I adult and Level II pediatric trauma center. Beth Israel Deaconess Medical Center is a major teaching hospital of Harvard Medical School, a tertiary care center and Level I trauma center. Seton Dell Medical School Stroke Institute serves an 11-hospital (Seton Family of Hospitals) network in central Texas, including a Level I trauma center and pediatric trauma center. Stony Brook University Hospital is a suburban, tertiary care, Level I trauma center. Brigham and Women’s Hospital is a 793-bed teaching affiliate of Harvard Medical School, a tertiary referral center and a Level I trauma center.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







