Fig. 19.1
Transthoracic echocardiogram documenting an aortic valve vegetation and severe aortic insufficiency in the parasternal long axis view and the 5-chamber apical view. Continuous wave Doppler signal of the aortic valve with a very short pressure half time suggestive of severe aortic insufficiency
Question
How should this patient’s native valve infective endocarditis (IE) be managed?
Answer
Antimicrobial therapy and aortic valve replacement.
All patients with IE should be initiated on early empiric guideline-recommended antibiotic therapy, and antimicrobials should be further guided by culture and sensitivities. Patients with severe mitral or aortic insufficiency causing congestive heart failure should be referred for early cardiac surgery to repair or replace the incompetent valve. Prior to surgery, this patient underwent a coronary CT scan, which reported a calcium score of 0. This test was done instead of a coronary angiogram to reduce the risk of dislodging the vegetation. A dental consultation excluded an oral abscess source. On post-admission day 2, he developed shock (blood pressure 70/20 mmHg) and flash pulmonary edema (Fig. 19.2). The patient was stabilized with non-invasive mechanical ventilation and vasopressors. Dopamine was selected to increase his blood pressure and his heart rate, with the intent of shortening diastolic filling time [1]. He underwent an emergent aortic valve replacement with a bioprosthetic valve. He was extubated and transferred to the surgical ward on post-operative day 2, and discharged home on post-operative day 6. An echocardiogram prior to discharge showed a normally functioning aortic bioprosthesis with no signs of infective endocarditis. Ceftriaxone was continued for a total of 6 weeks.
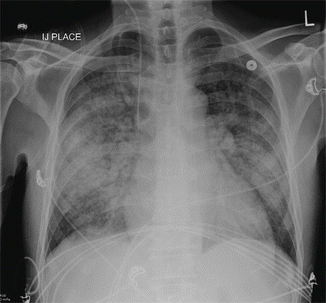

Fig. 19.2
Chest x-ray on post-admission day 2 showing severe pulmonary edema
Principles of Management
Epidemiology
The incidence of infective endocarditis is between 3 and 10 episodes per 100,000 person-years with a peak incidence during the ages of 70–80 of 14.5 episodes per 100,000 person-years [2–5]. Risk factors for IE include advanced age, poor dentition, injection drug use, structural heart disease (specifically valvular and congenital heart disease), the presence of prosthetic heart valves, and the presence of an intravascular catheter [6–10]. The most common micro-organisms responsible for native valve IE in order of likelihood are listed in Table 19.1 [11].
Microorganism | Frequency (%) |
|---|---|
Staphylococcus aureus | 31 |
Coagulase-negative staphylococcus | 11 |
Viridans group streptococci | 17 |
Streptococcus bovis | 6 |
Other streptococci | 6 |
Enterococcus species | 10 |
HACEK organisms | 2 |
Fungi/yeast | 2 |
Polymicrobial | 1 |
Negative cultures | 10 |
Other | 4 |
Diagnosis
Modified Duke criteria incorporate patient risk factors, physical exam findings, laboratory studies, and echocardiographic imaging to diagnose IE (Table 19.2) [12]. Importantly, three sets of peripheral venous blood cultures (aerobic and anaerobic) should be obtained from two sites (spatial separation) at 30 min intervals (temporal separation) prior to initiating antimicrobial therapy [1, 2]. Cultures should be investigated for typical and fastidious (e.g. HACEK organisms) pathogens. A transthoracic echocardiogram (TTE) is the first recommended imaging test in IE [1, 2]. Recognizing the TTE sensitivity of 40–63 % and specificity of 98 % [13, 14], a follow-up transesophageal echocardiogram (TEE) is recommended when: the TTE is non-diagnostic, the TTE is negative with a high index of suspicion of IE, structural cardiac complications are suspected, the patient has a prosthetic heart valve or an intra-cardiac device, or there is Staphylococcus aureus bacteremia [1, 2]. The reported sensitivity of TEE is 90–100 % [14, 15]. The reported negative predictive value of the original Duke Criteria is 92 % [16].
Table 19.2
Modified Duke criteria for infective endocarditis
Major criteria | |
1. Blood cultures positive for IE | |
a. Typical IE microorganism from two separate blood cultures | |
– Viridans streptococci | |
– Streptococcus bovis | |
– HACEK group | |
– Community-acquired enterococci | |
b. Microorganism consistent with IE from persistently positive blood cultures | |
– Two blood cultures drawn > 12 h apart | |
– All of three or a majority of ≥ 4 separate blood cultures (with first and last sample drawn at least 1 h apart) | |
c. Single positive blood culture for Coxiella burnetii or phase 1 IgG antibody titer > 1:800 | |
2. Evidence of endocardial involvement | |
a. Echocardiography positive for IE | |
– Vegetation | |
– Abscess | |
– New partial dehiscence of prosthetic valve | |
b. New valvular regurgitation | |
Minor criteria | |
1. Predisposition | |
– Predisposing heart condition | |
– Injection drug use | |
2. Fever – temperature > 38° Celsius | |
3. Vascular phenomena | |
– Major arterial emboli | |
– Septic pulmonary infarcts | |
– Mycotic aneurysm | |
– Intracranial haemorrhages | |
– Conjunctival haemorrhages | |
– Janeway lesions | |
4. Immunologic phenomena | |
– Glomerulonephritis | |
– Osler’s nodes | |
– Roth’s spots | |
– Rheumatoid factor | |
5. Microbiological evidence | |
– Positive blood culture but does not meet a major criterion | |
– Serological evidence of active infection with microorganism consistent with IE | |
Diagnosis | |
Definite IE | Possible IE |
2 major criteria | 1 major and 1 minor criteria |
1 major and 3 minor criteria | 3 minor criteria |
5 minor criteria | |
Antimicrobial Therapy
For acutely ill patients, an empiric antibiotic regimen of intravenous vancomycin, gentamicin, and ciprofloxacin or amoxicillin-clavulanate and gentamicin has been recommended [2]. However, initial empiric therapy should take into account local patterns of antibiotic resistance. In patients with prosthetic valves within 1 year of surgery, empiric therapy with vancomycin, gentamicin, and rifampin is recommended; rifampin should be initiated once cultures have cleared so as to reduce development of resistance [2]. Antimicrobial therapy and duration should be tailored to the specific organism and sensitivity according to published guidelines. The duration of therapy consists of a minimum of 4–6 weeks of intravenous antibiotics; the duration should be guided by guideline organism-specific recommendations [2].
Indications for Surgery
Cardiac surgery is recommended in the treatment of IE in the following situations [1, 17]: (1) aortic or mitral valve obstruction or regurgitation with heart failure, shock, severe regurgitation, or echocardiographic evidence of hemodynamic deterioration (early mitral valve closure or pulmonary hypertension); (2) locally uncontrolled infection or extension (abscess, fistula, aneurysm, heart block, or enlarging vegetation); (3) fungal, multidrug resistant, or highly resistant organisms; (4) Persistent bacteremia > 5–10 days despite appropriate antimicrobial therapy; (5) recurrent embolization with persistent vegetations; (6) vegetation > 15 mm; (7) prosthetic valves with relapsing infections.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







