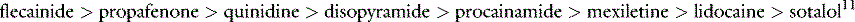Chapter 18 Management of cardiac arrhythmias
CARDIAC ELECTROPHYSIOLOGY
The electrophysiological properties of cardiac cells are important in understanding cardiac arrhythmias and their management. Cardiac cells undergo cyclical depolarisation and repolarisation to form an action potential. The shape and duration of each action potential are determined by the activity of ion channel protein complexes on the myocyte surface. These highly selective ion channels determine the rate of ion flux which in turn determines the magnitude and rate of change of myocyte membrane potential. Many of these ion channels are the molecular targets for antiarrhythmic drugs.
Ion channel function can be affected by:
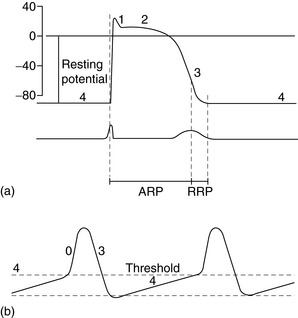
The spectrum of cardiac action potentials varies from fast-response cells – conducting and contractile myocytes (Figure 18.1a) – to slow-response cells of pacemaker myocytes – sinoatrial (SA) and atrioventricular (AV) nodes (Figure 18.1b). Fast myocytes lose their characteristic action potential and behave more like slow myocytes when ischaemic. The action potential is divided into five phases, as follows.
PHASE 0
In fast myocytes (Figure 18.1a) rapid depolarisation occurs due to activation of voltage-dependent Na+ channels. Activation is initiated in an all-or-none response once the threshold is reached. The Na+ channels are inactivated as membrane potential rises to +30 mV and remain inactivated until repolarisation occurs. Rapidity of depolarisation determines speed of conduction. In slow-response myocytes depolarisation does not involve Na+ channels and the slower rate of depolarisation is due to a slow inward Ca2+ current via L- and T-type voltage-dependent Ca2+ channels.
PHASE 1
Early rapid incomplete repolarisation to approximately 0 mV occurs due to activation of transient outward current from ITO1 and ITO2 K+ channels. Slow myocytes do not exhibit phase 1 or 2 characteristics (Figure 18.1b).
PHASE 4
This is a stable electrical state in fast non-pacemaker myocytes. In slow pacemaker myocytes the resting membrane potential (RMP) slowly depolarises until the action potential threshold is reached (Figure 18.1b). This inward or pacemaker current is due to If K+ channels.
Fast-response and slow-response myocytes also have important differences in properties of refractoriness. In fast myocytes, Na+ channels are progressively reactivated during phase 3 repolarisation as the membrane potential becomes more negative. When an extra stimulus occurs during phase 3, the magnitude of the resulting inward Na+ current and likelihood of impulse propagation depend on the number of reactivated Na+ channels. Refractoriness is therefore determined by the voltage-dependent recovery of Na+ channels. The absolute refractory period (Figure 18.1) is that minimum time needed for recovery of sufficient Na+ channels for a stimulus to result in impulse propagation. However, once propagation in fast myocytes occurs, conduction velocity is normal. In contrast, slow-response or Ca2+ channel-dependent myocytes exhibit time-dependent refractoriness. Even after full repolarisation further time is needed before all Ca2+ channels are reactivated. Stimuli during this period produce reduced Ca2+ current and the propagation velocity of any resulting impulse is reduced. The conduction velocity independence of premature action potentials with fast-response myocytes is lost in the setting of Na+ channel-blocking drugs or ischaemia because they behave increasingly like slow-response myocytes with resulting slowed impulse conduction.
GENETIC BASIS TO ARRHYTHMIA1
MOLECULAR BASIS TO ARRHYTHMIA1
Tachycardic remodelling of the atrium is associated with:
Heart failure is associated with:
Myocardial infarction scar produces:
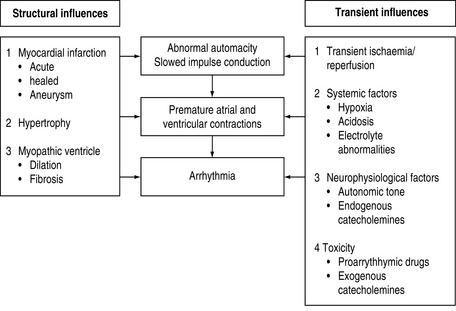
ARRHYTHMOGENIC MECHANISMS2–4
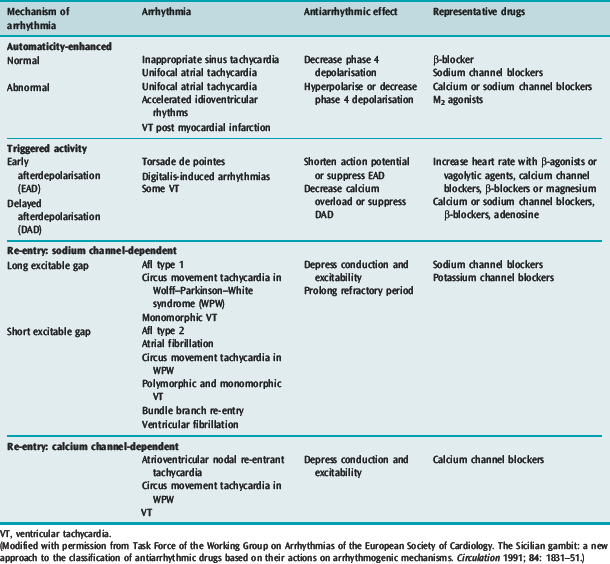
Many factors in isolation or combination give rise to the substrate of arrhythmogenesis (Figure 18.2). Arrhythmia may arise from abnormalities of impulse generation or conduction. Table 18.1 demonstrates the relationship between mechanism and type of arrhythmia, and desired antiarrhythmic effect.
ABNORMAL IMPULSE GENERATION (Table 18.2)
ENHANCED NORMAL AUTOMATICITY
Table 18.2 Causes of abnormal impulse generation
| Enhanced normal automaticity | Adrenergic stimulation |
| Abnormal automaticity | Ischaemia |
| Early afterdepolarisations | Hypoxia |
| Hypercapnia | |
| Catecholamines | |
| Class IA antiarrhythmic drugs | |
| Class III antiarrhythmic drugs | |
| Other drugs that prolong repolarisation | |
| Delayed afterdepolarisations | Digoxin toxicity |
| Increased intracellular Na+ | |
| Decreased extracellular K+ | |
| Increased intracellular Ca2+ | |
| Intracellular Ca2+ overload | |
| Myocardial infarction | |
| Reperfusion after ischaemia |
TRIGGERED ACTIVITY
ABNORMAL IMPULSE CONDUCTION5
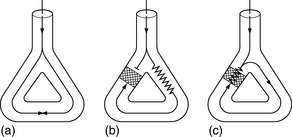
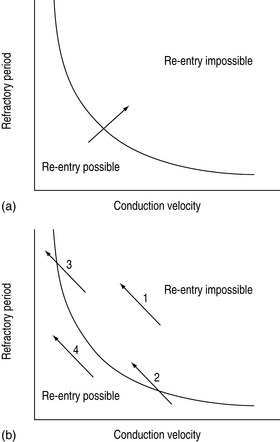
Abnormal impulse conduction may cause an arrhythmia by the phenomena of re-entry. Re-entry describes the re-excitation of an area or entire heart by a circulating impulse. Although the classic ‘bifurcating Purkinje fibre’ model of Schmitt and Erlanger has given way to a much more complex picture, the essential electrophysiological requirements for re-entrant excitation remain. Requirements for re-entry are (Figure 18.3):
When these properties are present, the chance of a circulating impulse producing re-entrant excitation depends on pathway geometry, the electrical properties and length of the depressed area and conduction velocity within each component. The segment of the re-entry pathway that is initially refractory and therefore blocks conduction down one limb and recovers in time to conduct the return impulse is termed the ‘excitable gap’. Therefore, the generation and subsequent maintenance of a circuit depend on this excitable gap of non-refractory tissue circulating between the advancing depolarising wave front and the repolarising tail. The resulting re-entrant impulse can be self-terminating, causing ectopic beats, or lead to atrial or ventricular tachyarrhythmias.
Re-entry may be terminated by:
The cellular properties that lead to impaired conduction include:
ELECTROLYTE ABNORMALITIES AND ARRHYTHMIA6
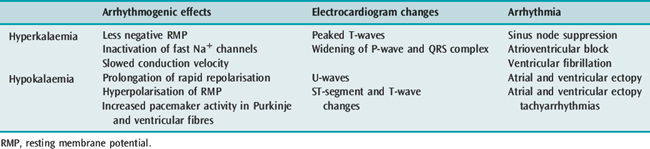
POTASSIUM
Hyper- and hypokalaemia both cause arrhythmia mediated by the resultant changes in RMP (Table 18.3). In ischaemia, hyperkalaemia at the local tissue level caused by a pathological extracellular shift of K+ is the major factor contributing to ventricular arrhythmia in this setting. In hypokalaemia, the dispersion of pacemaker activity and the effect on repolarisation are similar to the electrophysiological effects of cardiac glycosides and β-adrenergic agonists, and it is not surprising that a combination of these factors is associated with an increased incidence of arrhythmia. The increased risk of death in hypertensive patients treated with thiazide diuretics has been attributed to hypokalaemic (and possibly hypomagnesaemic)-induced arrhythmia (Multiple Risk Factor Intervention Trial). Thiazide-induced hypokalaemic ventricular ectopy is worsened by exercise.7 Hypokalaemia is associated with VF and VT following acute myocardial infarction (AMI). The increased incidence of VF/VT with a serum K+ less than 3.5 mmol/l is clearly established and the probability of VT increases as the serum K+ decreases. During AMI the incidence of VF/VT was 15% at 4.5 mmol/l, 38% at 3.5 mmol/l, 55% at 3.0 mmol/l and 67% at 2.5 mmol/l.8
MAGNESIUM
The antiarrhythmic properties of Mg2+ are clearly established but a causal relationship between hypomagnesaemia and arrhythmia is largely circumstantial. Decreased extracellular Mg2+ by itself has little effect on the electrophysiological properties of myocytes or the ECG. Hypomagnesaemia has been implicated in the genesis of VT/VF in patients with hypertension and heart failure receiving thiazide or loop diuretics, acute alcohol intoxication or withdrawal and possibly with AMI. The product of K+ and Mg2+ is the best predictor of arrhythmia in hypertensive patients taking thiazide diuretics.9
AUTONOMIC NERVOUS SYSTEM AND VENTRICULAR ARRHYTHMIA10
PROARRHYTHMIC EFFECTS OF ANTIARRHYTHMIC DRUGS11,12
Concomitant proarrhythmia with the use of antiarrhythmic drugs is increasingly recognised. The ‘quinidine syncope’ due to VF and polymorphic VT at therapeutic concentrations was also seen with disopyramide. The Cardiac Arrhythmia Suppression Trial (CAST) clearly defined the magnitude of this deleterious side-effect in drugs that were previously perceived to be of benefit.13 This study, which involved flecainide, encainide and morizicine (a class IA drug), was terminated early because of adverse outcome in the flecainide and encainide groups (relative risk of arrhythmic death or non-fatal cardiac arrest of 3.6, 95% confidence interval (CI) 1.7–8.5). Proarrhythmia is reported between 5.9% and 15.8% depending on agent, clinical setting and definition of proarrhythmia, and now considered ubiquitous with all antiarrhythmic drugs.
Proarrhythmia has been defined as an increase in frequency of ventricular ectopic beat (VEB) or aggravation of the target arrhythmia on Holter monitor or exercise test. Manifestations of proarrhythmia not only include VEB, monomorphic and polymorphic VT and VF, but also bradyarrhythmias and Afl with 1:1 AV conduction. Most proarrhythmic events occur soon after starting the drug, but late arrhythmias are also a significant problem.
The mechanism of drug proarrhythmia is probably via both slowing of conduction and abnormal automaticity. Paradoxically slowing conduction, which may block a re-entry circuit, may also create the very substrate needed for re-entry, unidirectional block and an excitable gap. The existence of a re-entrant circuit requires the circulating wave front of the impulse not to catch up with the refractory tissue behind the tail. Re-entry is more likely to occur with a shorter refractory period and reduced conduction velocity (Figure 18.4).11
Antiarrhythmic drugs are effective at suppressing abnormal automaticity, with the exception of triggered automaticity due to EAD. Class IA, class III and many non-antiarrhythmic drugs can produce proarrhythmia via EAD. These drugs increase not only the frequency of EAD, but also the likelihood of them leading to triggered tachyarrhythmias. Slowing repolarisation, which leads to QT prolongation and slower heart rate, is central to this increased frequency and sensitivity to EAD. EAD manifests as prominent and bizarre T-U waves on the ECG and, if triggered activity results, VEB and ventricular tachyarrhythmias may occur. Torsade de pointes is the classical resulting arrhythmia, although less classical polymorphic VT and VF result. Risk of proarrhythmia via this mechanism correlates with the degree of QT prolongation.
Antiarrhythmic drug proarrhythmia is facilitated by several factors, which are frequently found in patients on antiarrhythmic drugs or with heart disease (Table 18.4).
Table 18.4 Factors facilitating antiarrhythmic drug proarrhythmia
| Toxic blood levels due to excessive dose or reduced clearance from old age, heart failure, renal disease or hepatic disease |
| Severe left ventricular dysfunction. Ejection fraction less than 35% |
| Pre-existing arrhythmia or arrhythmia substrate |
| Digoxin therapy |
| Hypokalaemia or hypomagnesaemia |
| Bradycardia |
| Combinations of antiarrhythmic drugs and concomitant drugs with similar toxicity |
(Adapted from Campbell TJ. Proarrhythmic actions of antiarrhythmic drugs: a review. Aust NZ J Med 1990; 20: 275–82, with permission.)
MANAGEMENT OF THE PATIENT WITH A CARDIAC ARRHYTHMIA
INVESTIGATIONS
A 12-lead ECG should be recorded with a longer rhythm strip (usually lead II or V1). If P-waves are not visible, atrial activity may be recorded using an oesophageal electrode or pacing lead, or via a central venous catheter or the right atrial injectate port of a pulmonary artery catheter, using 20% saline and a bedside monitor.14
EPS, which involves invasive electrophysiological testing with programmed electrical stimulation, attempts to reproduce the spontaneously occurring arrhythmia.15,16 EPS is not clearly superior to Holter monitoring in evaluating drug treatment for ventricular arrhythmias.
Other investigative techniques being studied include signal-averaged ECG, heart rate variability and electrical alternans measurement.10,17
MANAGEMENT OF SPECIFIC ARRHYTHMIAS
Treatment has two aspects: acute termination of the arrhythmia and long-term prophylaxis. The decision whether to treat depends on the rhythm diagnosis, haemodynamic consequences, aetiology of the arrhythmia and the prognosis (e.g. risks of sudden death or long-term complications).
PREMATURE VENTRICULAR ECTOPIC BEATS
ECG
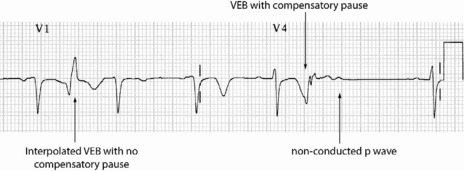
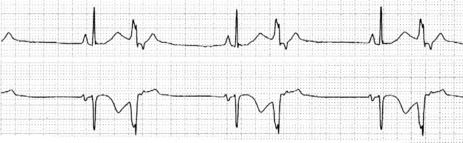
Occasionally VEB may not produce any pause, and are said to be interpolated (see Figure 18.6). Interpolated VEB occur when the background sinus rhythm is slow. The retrograde conduction into the AV node renders it partially refractory to the next impulse and its conduction through the AV node is slowed and the PR interval is prolonged. A VEB following each sinus beat is ventricular bigeminy (Figure 18.7). Ventricular trigeminy refers to recurring sequences of a VEB followed by two sinus beats. Two VEB in succession are a couplet (Figure 18.8), and three, a triplet.
CLINICAL
Even when frequent, complex, or in short runs of non-sustained VT, VEB are not associated with risk of sudden death in asymptomatic healthy adults.18 However, there is increased risk of cardiovascular death with:
SUPRAVENTRICULAR TACHYCARDIAS22,23 (Table 18.5)
Table 18.5 Classification of supraventricular tachycardias
| Atrioventricular (AV) node-dependent |
| AV nodal re-entry tachycardia: re-entry within the AV node |
| AV re-entry tachycardia: re-entry includes accessory pathway between atria and ventricles |
| Accelerated idionodal rhythm: increased automaticity of AV nodea |
| AV node-independent |
| Atrial flutter: re-entry confined to atria |
| Atrial fibrillation: multiple re-entry circuits confined to atria |
| Unifocal atrial tachycardia: usually due to increased automaticity |
| Multifocal atrial tachycardia: increased automaticity or triggered activity |
| Others: sinus node re-entry tachycardia |
A clinically useful classification divides SVT into AV node-dependent and AV node-independent.
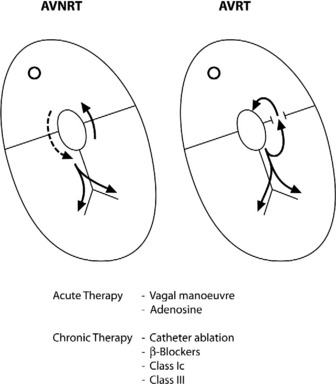
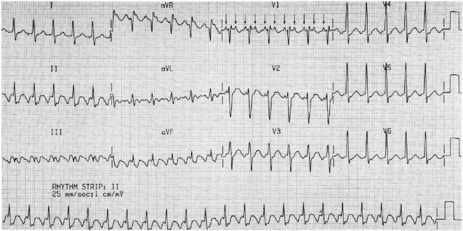
AV NODAL RE-ENTRY TACHYCARDIA (AVNRT)(Figure 18.9)
ECG
There is regular narrow-complex tachycardia (140–220 beats/min) with abrupt onset and termination. P-waves are not usually observed as they are buried in the QRS complexes (Figure 18.10).
TREATMENT
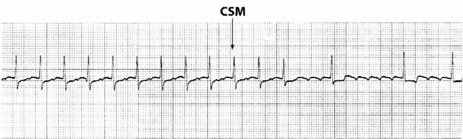
Vagal manoeuvres slow conduction through the AV node and may ‘break’ the tachycardia. If carotid sinus massage fails, adenosine is the drug of choice and nearly all AVNRT will revert with adenosine.24,25 Verapamil has been used in the past, but causes hypotension, which may be prolonged if cardiac function is depressed or patients are receiving β-adrenergic blockers. Sotalol, amiodarone and flecainide may also be effective but are rarely used. Rapid atrial pacing will usually terminate AVNRT but is rarely needed.
AV RE-ENTRY TACHYCARDIA (SEE Figure 18.9)
ECG
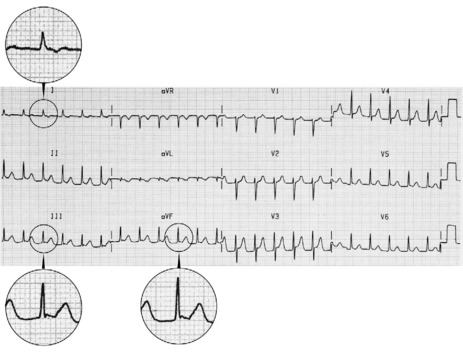
The ECG is similar to AVNRT. The length of the re-entry circuit is however greater, and the accessory AV pathway is some distance from the AV node. It therefore takes longer for the impulse to be conducted backwards to the atria, and so the retrograde P-wave usually occurs after the QRS, sometimes at some distance, and is inverted in leads II, III and aVF (Figure 18.11 and 18.12).
TREATMENT24,25
Acute treatment is identical to AVNRT, but verapamil should be avoided in WPW syndrome, as it may block the AV node, facilitating very rapid conduction to the ventricles via the accessory pathway.27
ACCELERATED IDIONODAL RHYTHM
ECG
There are narrow complexes on the ECG at a regular rate (60–130 beats/min) (Figure 18.13), often with independent atrial activity. With isorhythmic dissociation, P-wave is either fixed relative to the QRS complex (usually just after) or oscillates to and fro across the QRS in a rhythmical manner.
CLINICAL
It may be observed in normal persons, but is often associated with structural heart disease, especially following inferior myocardial infarction. Digoxin intoxication is another important cause.
UNIFOCAL ATRIAL TACHYCARDIA
ECG
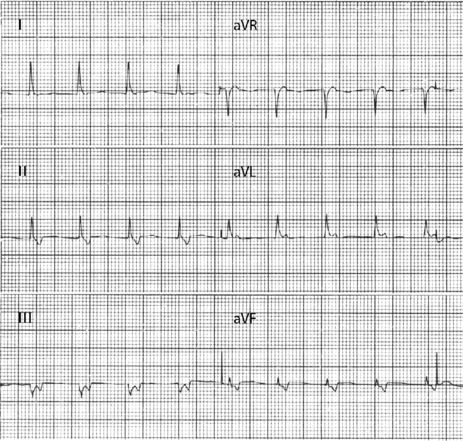
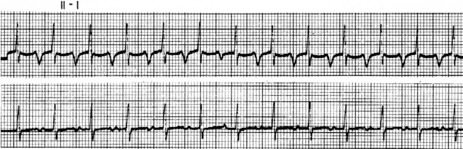
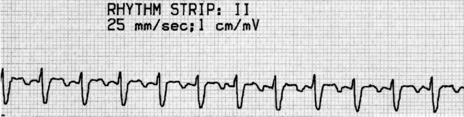
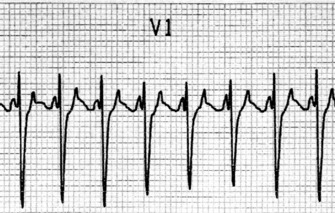
P-wave morphology is abnormal but monomorphic. Atrial rate is often 130–160 beats/min, and may occasionally exceed 200 beats/min. Atrial rate distinguishes unifocal atrial tachycardia from atrial flutter (Afl), with Afl greater than 250 beats/min. The QRS complexes will usually be narrow (Figure 18.14). AV block is common (Figure 18.15).
CLINICAL
Digitalis intoxication is the most common cause, especially when AV block is present. Other causes include myocardial infarction, chronic lung disease and metabolic disturbances.
MULTIFOCAL ATRIAL TACHYCARDIA28
ECG
There are irregular atrial rates, usually 100–130 beats/min, with varying P-wave morphology (at least three different P-wave morphologies and varying PR interval) and some degree of AV block (Figure 18.16). Most P-waves are conducted to the ventricles, usually with narrow QRS complexes.
TREATMENT
Treatment should correct the underlying cause (e.g. treatment of cardiorespiratory failure, electrolyte and acid–base abnormalities and theophylline toxicity). Spontaneous reversion is common, and few patients require antiarrhythmic therapy. Magnesium is the drug of choice for acute control.29 β-Blockers are probably more effective than diltiazem, but because of the common association of MAT with obstructive lung disease have limited utility.30 Digoxin and cardioversion are ineffective, which highlights the need to differentiate MAT from AF. Longer-term control is best achieved with diltiazem in patients with good left ventricular (LV) function and amiodarone in those without.
ATRIAL FLUTTER31
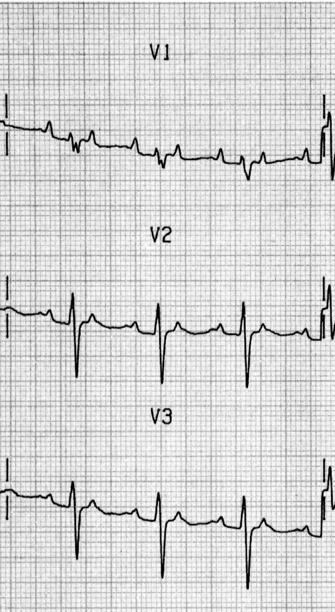
ECG
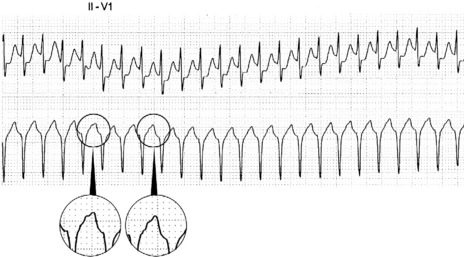
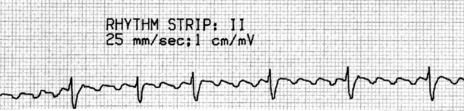
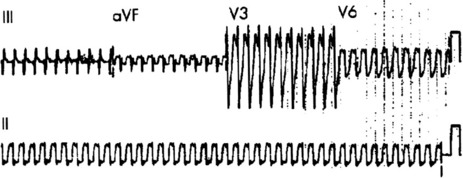
Afl waves (characteristic sawtooth appearance with no isoelectric baseline) are best seen in V1 (Figure 18.17) or aVF, but leads II and III may also be useful. The flutter waves are usually negative in aVF. Rapid QRS waves may obscure typical flutter waves, and vagal manoeuvres may unmask them (see Figure 18.5). AV conduction block (usually 2:1) is usually present, so that alternate flutter waves are conducted to the ventricles, with a ventricular rate close to 150 beats/min. Frequently flutter waves are not obvious and a ventricular rate of 150 beats/min leads to the presumption of Afl (Figure 18.18). Type II Afl results in greater atrial and ventricular rates (Figure 18.19). Treatment with drugs that affect AV node conduction may lead to higher degrees of AV block (Figure 18.20) and/or variable AV block with irregular QRS duration. Rarely, Afl with 1:1 conduction occurs. This is usually associated with sympathetic overactivity or class I antiarrhythmic drugs (which slow atrial discharge rate to 200 beats/min, thereby allowing each atrial impulse to be conducted) (Figure 18.21). QRS complexes are usually narrow, as conduction through the bundle branches is normal.
TREATMENT25
No drug will reliably terminate Afl, although ibutillide and dofetilide have been shown to be most likely to result in pharmacological reversion. Attempts at slowing ventricular rate by drugs that will increase the degree of AV block are worthwhile in the first instance. Drugs such as digoxin, diltiazem, β-adrenergic blockers, sotalol and amiodarone may be tried; the choice depends on LV function. Flecainide and procainamide may occasionally be effective at terminating Afl. However, class IA and IC drugs may lead to 1:1 AV conduction. Class I drugs should probably be avoided unless ventricular response has been slowed with calcium channel or β-adrenergic blocking drugs.
Anticoagulation guidelines are the same as that for AF, although there are less supporting data.
PREVENTION
Prevention is difficult. Drugs used include sotalol and amiodarone at low doses. Class IC agents (e.g. flecainide) may be used in patients without significant structural heart disease. Increasingly recurrent or refractory Afl may be cured by radiofrequency ablation to create a linear lesion between the inferior tricuspid annulus and the eustachian ridge at the anterior margin of the inferior vena cava to interrupt the re-entry circuit.26
ATRIAL FIBRILLATION32
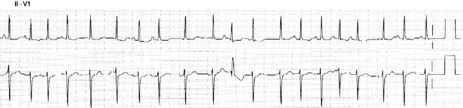
ECG
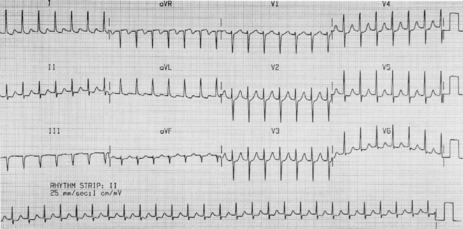
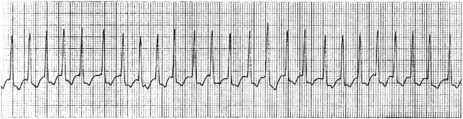
Atrial activity is chaotic with rapid (350–600 beats/min) and irregular depolarisations varying in amplitude and morphology (fibrillation waves). Ventricular response is irregularly irregular (Figure 18.22). Most atrial impulses are not conducted to the ventricles, resulting in an untreated ventricular rate of 100–180 beats/min. QRS complexes will usually be narrow. When the ventricular rate is very rapid or very slow, ventricular irregularity may be missed (Figure 18.23).
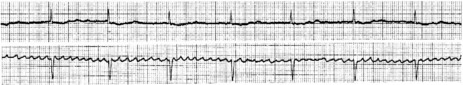
Figure 18.22 Atrial fibrillation. Irregular fibrillation waves with varying amplitude and morphology.
CLINICAL
TREATMENT25,34,35
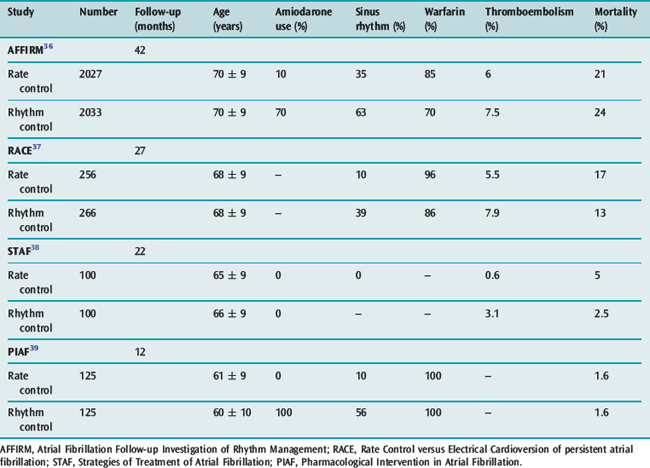
The goals of treatment include ventricular rate control, anticoagulation where appropriate and conversion to sinus rhythm. There is increasing evidence available on the ‘rate versus rhythm’ control debate. Results from several recent major studies have challenged the previous belief that achievement of sinus rhythm is important in the long term (Table 18.6). When comparing control of ventricular rate versus reversion to sinus rhythm no clear survival benefit is apparent. However composite end-points of death, stroke and recurrent hospilisation favour rate control only.36–39
The possible reasons why rhythm control has not been shown to be superior include:
However rhythm control (if possible) appears superior in patients with LV dysfunction, with both amiodarone and dofetilide reducing mortality when sinus rhythm is achieved.40,41 The paucity of data in younger patients (less than 60 years) favours initial attempts at rhythm control, particularly in those with structurally normal hearts, in the hope that progressive atrial electrical and anatomical remodelling is prevented.
RECENT ONSET OR PAROXYSMAL AF
VENTRICULAR RATE CONTROL
CONVERSION TO SINUS RHYTHM
Antiarrhythmic drugs or DC shock cardioversion can be used. The likelihood of short- and long-term success depends on the clinical situation. Conversion to sinus rhythm is more important in young patients and those with heart failure. Maintenance of sinus rhythm is problematic: sinus rhythm at 1 year is 60% with amiodarone and 40% with sotalol, and associated with significant drug cardiac and extracardiac toxicities. The risk of stroke and need for antithrombotic therapy due to frequent AF recurrences, which may be asymptomatic, remain. Achieving sinus rhythm (especially greater than 60 years) is less important than previously thought.36
DC SHOCK CARDIOVERSION
Critically ill patients who are septic, postoperative or on drugs such as catecholamines are likely to relapse.
ANTIARRHYTHMIC DRUGS
Quinidine is more effective than placebo, but increases mortality through proarrhythmia (generally class IA and IC antiarrhythmic drugs are contraindicated). Ibutilide and dofetilide are newer antiarrhythmic drugs with particular success at pharmacological cardioversion. Pretreatment with ibutilide increased DC shock cardioversion from 72% for placebo to 100%. In placebo failures, cross-over to ibutilide resulted in a 100% success rate with subsequent cardioversion. Ibutilide also resulted in reduction in DC shock energy required from 228 ± 93 J to 166 ± 80 J. However, ibutilide was associated with a 3% incidence of sustained polymorphous VT.45 Dofetilide appears to cardiovert AF and Afl pharmacologically in about a third of patients (intravenous (IV) better than oral, recent onset better than prolonged and Afl may be more responsive than AF). Dofetilide is far superior to placebo and sotalol, with similar recurrence rates to amiodarone.46
Other drugs currently used to promote onset of sinus rhythm and prevent AF relapse include amiodarone, sotalol, procainamide, flecainide and propafenone. Amiodarone was found to be superior in preventing AF recurrence with a recurrence rate of 35% compared to a recurrence rate of 63% for sotalol and propafenone.47
The factors dictating choice are:
When using amiodarone for prevention of AF recurrence there was an 18% incidence of adverse effects versus 11% for sotalol and propafenone.47
ATRIAL FIBRILLATION ABLATION THERAPY
Ablation techniques for AF have been continuously refined since the original Maze III surgical procedure which involved numerous atrial incisions to form a maze-like pattern of scarring, blocking propagation of arrhythmia. The ultility of this procedure was limited because it was surgical, with longer bypass times, postoperative bleeding and impaired atrial contractility. The magnitude of this original procedure was based on the belief that the entire atrium was involved in the initiation and maintenance of the fibrillatory conduction. This may be true for long-standing AF but paroxysmal AF appears to originate primarily at the junction of the left atrium and pulmonary veins. AF in 94% of patients is initiated by rapid discharges from one or more foci at or near the pulmonary vein orifices.48 Atrial tissue in this area has heterogeneous electrophysiological properties and there is also clustering of vagal inputs, which creates substrate for rapid discharges that initiate microre-entrant circuits or ‘rotors’. These high-frquency periodic rotors send spiral wave fronts of activation into surrounding atria. Localised ablation of a single dominant foci and rotor is inadequate as there are usually multiple foci.
Left atrial catheter (transatrial septum) AF ablation isolating all four pulmonary veins using radiofrquency is being heralded as the possible AF cure. Results are improving as all pulmonary veins are now isolated and the encircling lesion is clear of the pulmonary vein antrum (reducing pulmonary vein stenosis). Success rates of 81% (75–88%) free of AF and off drugs are reported. Success appears long-term as recurrence occurs early. A further 10–20% may become responsive to antiarrhythmic drugs which were previously ineffective. Repeating the procedure can increase success to > 90% with failure only in patients found to have extensive atrial scarring (predicting and excluding patients with this extensive atrial scarring is a major future challenge). Although not yet the universal cure the results are two- to threefold better than antiarrhythmic drugs alone.
Complication rates are also falling associated with:
Transient ischaemic attacks, strokes, tamponade/perforation and symptomatic pulmonary vein stenosis are all well below 1% respectively. Proarrhythmia resulting from re-entrant tachycardias from incomplete ablative lesions is more common. Some are advocating ablation as first-line treatment whereas most are selecting younger patients (less than 70 years) with paroxysmal AF for whom antiarrhythmic therapy has failed, left atrial diameter is less than 5 cm and ejection fraction is greater than 40%.26 Head-to-head studies comparing ablation and antiarrhythmic drugs are appearing with suggested survival benefit, improved quality of life, reduced adverse effects and cost-effectiveness after approximately 3 years with catheter AF ablation therapy.49,50
ANTICOAGULATION FOR CHRONIC ATRIAL FIBRILLATION
Consider for all patients, especially those with risk factors (Table 18.7).
Table 18.7 Prognostic factors for ischaemic stroke and systemic embolism in patients with atrial fibrillation
| High | Previous stroke, transient ischaemic attack, systemic embolism |
| Mitral stenosis | |
| Prosthetic heart valve | |
| Moderate | Age > 75 years |
| Left atrial size > 45 mm | |
| Hypertension | |
| Congestive cardiac failure | |
| Diabetes mellitus | |
| Left ventricular ejection fraction < 35% | |
| Low | Female |
| Age 65–74 years | |
| Coronary artery disease | |
| Thyrotoxicosis |
NON-VALVULAR ATRIAL FIBRILLATION
The risk of stroke is determined by CHADS2 score (assign 1 point for congestive heart failure, hypertension, age = 75 years and diabetes mellitus, and 2 points for stroke/TIA)51 (Tables 18.8 and 18.9).
Table 18.8 CHADS2 score stroke risk stratification in non-valvular atrial fibrillation
| Prognostic factor | Relative risk* | CHADS2 score |
|---|---|---|
| Congestive heart failure (ejection fraction < 35%) | 1.4 | 1 |
| History of hypertension | 1.6 | 1 |
| Age ≥ 75 years | 1.4 | 1 |
| Diabetes mellitus | 1.7 | 1 |
| Stroke or transient ischaemic attack in past | 2.5 | 2 |
CHADS2, congestive heart failure, hypertension, age = 75 years and diabetes mellitus, and 2 points for stroke/transient ischaemic attack.
* Relative risk without any antithrombotic treatment compared to atrial fibrillation patients without these prognostic factors.
Table 18.9 The adjusted annual stroke rate in non-valvular atrial fibrillation without any antithrombotic treatment
| CHADS2 score | Adjusted stroke rate | |
|---|---|---|
| %/year | 95% CI | |
| 0 | 1.9 | 1.2–3.0 |
| 1 | 2.8 | 2.0–3.8 |
| 2 | 4.0 | 3.1–5.1 |
| 3 | 5.9 | 4.6–7.3 |
| 4 | 8.5 | 6.3–11.1 |
| 5 | 12.5 | 8.2–17.5 |
| 6 | 18.2 | 10.5–27.4 |
CHADS2, congestive heart failure, hypertension, age = 75 years and diabetes mellitus, and 2 points for stroke/transient ischaemic attack; CI, confidence interval.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree