Fig. 7.1
12-lead EKG revealing sinus bradycardia
Question
What do you think is causing her hyperglycemia and what therapeutic intervention(s) could be considered at this point to improve her hemodynamic stability?
Answer
Impaired insulin secretion due to calcium channel blocker inhibition of pancreatic Beta cells, coupled with increased stress-mediated glucose mobilization account for her hyperglycemia. High-dose insulin euglycemic therapy should be considered at this point. Her metabolic acidosis is secondary to both a lactic acidosis from impaired tissue perfusion, and ketoacidosis similar to that seen with DKA due to relative hypoinsulinemia.
Based on her weight of 50 kg, an insulin bolus of 50 units IV was given followed by an infusion of 50 units/h. Approximately 45 min after initiation of the insulin infusion, her pulse normalized to 65 and her blood pressure improved enough to gradually wean the norepinephrine drip. Her glucose was monitored every 30 min, and ultimately she did require a continuous dextrose infusion to maintain serum glucose concentrations above 150 mg/dL. Her metabolic acidosis improved over the course of several hours, as did her serum lactate. Serum electrolytes were monitored closely. She developed mild hypokalemia but repletion was not necessary. Approximately 24 h following admission, the insulin infusion was discontinued and her vitals remained stable. She was discharged the next morning to an inpatient psychiatric facility.
Principles of Management
Gastrointestinal Decontamination
Activated charcoal effectively binds calcium channel blockers and should be administered in patients with stable airways and preserved mental status who present within the first 2 h following ingestion. Although clinical evidence supporting the use of multi-dose activated charcoal is lacking, it is reasonable to consider repeat charcoal dosing when sustained-release preparations are involved [2, 5]. Gastric lavage is generally not recommended since the procedure may increase vagal tone, exacerbate hemodynamic instability, and can provoke cardiac arrest [6, 7]. Whole bowel irrigation may be considered in cases of sustained-release preparations, or when decontamination is delayed beyond a time-frame where activated charcoal would be of benefit [6]. Whole bowel irrigation should be avoided, however, in patients with depressed mental status, ileus, airway compromise, or hemodynamic instability [8].
Hemodynamic Support
The initial management of hypotension due to calcium channel blocker toxicity should include aggressive intravenous fluid resuscitation. In more severely poisoned patients, several therapeutic options may also be considered.
Calcium Salts
The use of calcium gluconate and calcium chloride in the management of calcium channel blocker toxicity seems intuitive. Increased serum calcium concentrations would be expected to overcome calcium channel blockade via a gradient effect, thereby improving myocardial contractility [6]. Animal studies suggest the use of calcium confers both hemodynamic benefits and improves mortality. Human studies are limited to case series, and the reported benefits are inconsistent [7, 9]. The response seen with calcium is often transient, and repeat dosing may be needed [2]. Despite conflicting evidence regarding benefit, use is generally recommended and adverse effects are rare.
Glucagon
Glucagon increases intracellular cyclic-AMP and has been shown in animal models to have positive inotropic and chronotropic effects. Furthermore, glucagon has been shown, in some cases, to reverse 2nd and 3rd degree heart blocks [10]. Evidence for its efficacy in humans is limited to case reports, and treatment failures have been described [2, 7]. Glucagon dosing is not well-established, but an initial dose of 3–5 mg IV followed by a continuous infusion has been suggested, with an additional dose of 4–10 mg IV 5 min after the initial dose if no response is achieved [6].
Atropine
Atropine may be considered for symptomatic bradycardia, but is often ineffective in the setting of severe poisonings. Standard advanced cardiac life support (ACLS) dosing guidelines should be used. Because of its anticholinergic effects on GI motility, it’s use may potentiate absorption of sustained-release calcium channel blocker formulations [6].
Vasopressor Support
When hemodynamic stability cannot be achieved through the use of fluid resuscitation and other initial pharmacologic strategies, vasopressor support may be necessary. Norepinephrine, dopamine, epinephrine, isoproterenol, dobutamine, and phenylephrine have all been used to achieve improvements in blood pressure, and no studies have demonstrated the superiority of one agent over another [2]. Multiple agents may be required simultaneously to achieve hemodynamic stability, and some have reported use of vasopressor doses far in excess of what would typically be considered the referenced maximum [4]. Although improvements in blood pressure are typically achieved, one must be mindful that the increase in systemic vascular resistance will also increase afterload. This may paradoxically lead to an undesirable decrease in cardiac output, as well as an increase in the cardiac oxygen requirement in an already energy-depleted myocardium [3, 6].
High-Dose Insulin Therapy
In recent years, high-dose insulin therapy for the treatment of calcium channel blocker poisonings has gained increasing attention. Calcium channel blockers directly inhibit the calcium channel-mediated release of insulin by the pancreas, leading to systemic hypoinsulinemia (Fig. 7.2). Because carbohydrates are the preferred metabolic substrate of myocardial cells when under duress, the impairment of intracellular glucose transport secondary to insulin depletion further worsens cardiac contractility already impaired by the calcium channel blockers themselves [11]. High-dose insulin restores myocardial glucose utilization and corrects systemic ketoacidosis when present. Insulin has also been shown to have direct positive inotropic effects on myocytes [11]. Furthermore, insulin has been shown to induce vasodilation which improves microvascular perfusion in tissues including the myocardium [12]. Insulin also promotes increased catecholamine sensitivity [11]. The combination of these effects may ameliorate the cardiogenic shock seen with calcium channel blocker toxicity.
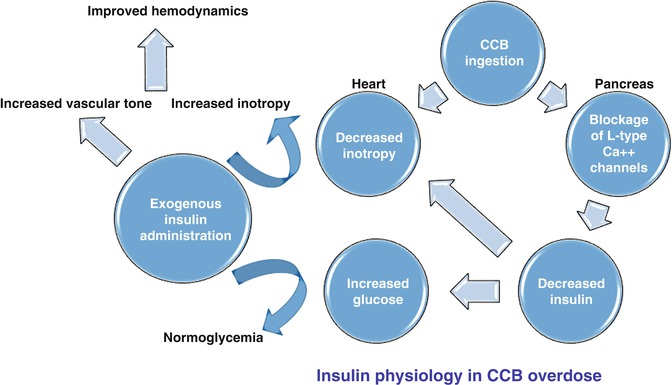

Fig. 7.2
CCB calcium channel blocker. L-type Ca++ channels: voltage-gated calcium channels
Animal models suggest the mortality benefit from high-dose insulin therapy is superior to that seen with calcium salts, glucagon, and vasopressors. One human observational study showed that high-dose insulin therapy resulted in a >10 mmHg sustained increase in systolic blood pressure in all patients receiving high dose insulin boluses and infusions, and numerous case series and case reports detail beneficial hemodynamic responses [2, 13, 14].
Although definitive dosing guidelines have not been established, most recommend an initial insulin bolus be given at a dose of 1 U/kg IV followed by an infusion 1 U/kg/h which may be titrated upward [6]. Higher bolus doses of 10 U/kg and infusions up to 22 U/kg/h have been reported [3]. The clinical response to high-dose insulin may take 15–60 min. Many sources advocate initiation of high-dose insulin therapy very early on in the management of these patients (before they become unstable) [6, 14, 15]. Serum glucose should be monitored every 30 min during the initial course of treatment and supplemental dextrose should be administered, although supplementation may not be necessary if the initial glucose exceeds 300 mg/dL [6]. Mild hypokalemia should be anticipated due to intracellular shifts and may actually augment myocardial cellular function by improving intracellular calcium transport. Potassium repletion should be considered if serum levels fall below 2.8–3.0 mEq/L [12].
Invasive Circulatory Support
The use of temporary transcutaneous and transvenous pacemakers, and intra-aortic balloon pump counter pulsation therapy have been described in the setting of severe bradycardia and high-degree atrioventricular blocks in calcium channel blocker poisonings when medical management fails to adequately reverse cardiogenic shock [2, 6, 7]. At times, obtaining successful pacemaker capture may be difficult in these patients. Furthermore, improvement in hemodynamics may be variable owing to the persistent impaired cardiac inotropy, and it is unclear based on case reports whether pacemakers improve clinical outcomes [7, 16]. There are reports describing the successful use of intra-aortic balloon pumps and extracorporeal life support in the setting of refractory shock due to severe calcium channel blocker poisonings, and these techniques may be considered on a case by case basis when other treatment strategies fail [17–19].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






