Clinical factor
Pretest probability of an UGIB
Likelihood ratio
Posttest probability of an UGIB
Melena on exam
63 %
25
98 %
BUN/sCr >30
63 %
7.5
93 %
Prior history of UGIB
63 %
6.2
91 %
Subjective melena
63 %
5.5
90 %
Positive NG lavage
63 %
4.7
89 %
Table 61.2
Clinical factors that decrease the probability of an UGIB
Clinical factor | Pretest probability of an UGIB | Likelihood ratio | Posttest probability of an UGIB |
|---|---|---|---|
Clots in Stool | 63 % | 0.05 | 8 % |
Prior history of LGIB | 63 % | 0.17 | 22 % |
Lack of subjective melena | 63 % | 0.22 | 27 % |
Negative NG lavage | 63 % | 0.6 | 51 % |
Risk Stratification
Risk assessment is uniformly recommended for all UGIB patients to determine the timing of endoscopy and the level of care the patient will need. Factors such as a history of malignancy or cirrhosis, syncope, tachycardia, shock, hemoglobin <8 g/dL, BUN >90 mg/dL and white blood cell count >12,000 all increase the probability of a severe gastrointestinal bleed that will have active bleeding on endoscopy [1]. The Glasgow-Blatchford Score (GBS) is a well-validated clinical score that was developed using many of these clinical factors (Table 61.3 ) to predict mortality and the need for a clinical intervention (blood transfusion, endoscopic therapy, or surgery). A score ≤2 safely predicts patients that can be managed as an outpatient with a sensitivity ≥98 %. A higher score represents higher risk patients that should receive an endoscopy within 24 h. The Rockall score was developed with the same purpose, but has two main drawbacks. It requires endoscopic information, which is not available on the initial evaluation, and it does not perform as well as the GBS score [1]. For these reasons many professional guidelines recommend using the GBS score [3].
Table 61.3
Glasgow-blatchford score
Admission risk marker | Score value |
|---|---|
Blood urea nitrogen (mmol/L) | |
6.5–7.9 | 2 |
8.0–9.9 | 3 |
10.0–24.9 | 4 |
≥25.0 | 6 |
Hgb (g/dl) for men | |
12–12.9 | 1 |
10–11.9 | 3 |
<10 | 6 |
Hgb (g/dl) for women | |
10–11.9 | 1 |
<10 | 6 |
Systolic blood pressure (mm Hg) | |
100–109 | 1 |
90–99 | 2 |
<90 | 3 |
Pulse ≥100 (per min) | 1 |
Presentation with melena | 1 |
Presentation with syncope | 2 |
Hepatic disease | 2 |
Cardiac failure | 2 |
Blood Transfusion
Blood transfusions should generally be withheld until the hemoglobin is ≤7 g/dL [4, 5]. In 2013, a large randomized control trial (RCT) found that patients treated with a restrictive strategy (Hgb <7 g/dL) had better outcomes, including a 40 % relative reduction in mortality, less bleeding, less surgery, fewer transfusion reactions, and fewer cardiac complications. Exsanguinating patients were not enrolled and the protocol allowed blood transfusions in symptomatic patients. Consequently it is important to carefully weigh the clinical situation in the decision to transfuse [6].
Post-endoscopic Intensive Proton Pump Inhibitor Therapy
Patients with a bleeding peptic ulcer and high-risk lesions on endoscopy have improved outcomes when treated with 3 days of intensive proton pump inhibitor (PPI) therapy consisting of an intravenous bolus followed by a continuous infusion for 72 h. A 2006 Cochrane Review found patients with a high-risk peptic ulcer lesion who received PPI therapy had lower rates of rebleeding and surgery. Patients with very high-risk lesions had an even greater benefit from PPI therapy, including a mortality benefit [7]. Lesions are graded using the Forrest classification, and each lesion has a specific rebleeding, surgery and mortality risk (Fig. 61.1). Intensive PPI therapy will have the greatest impact on high-risk lesions because they have the worst outcomes. Low-risk lesions have excellent outcomes so intensive PPI therapy is unlikely to be beneficial. The benefits of intensive PPI therapy are so clear that professional guidelines unanimously recommend them in patients with high-risk peptic ulcer lesions [3–5] (Video 61.1).
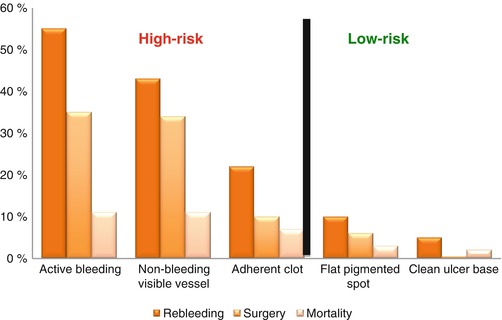

Fig. 61.1
Forrest classification. The risk of rebleeding, surgery and mortality based on the Forrest classification of the ulcer seen on endoscopy. Only high-risk lesions benefit from intensive PPI therapy (Data from Barkun et al. [4])
Evidence Contour
Nasogastric Lavage
Significant controversy exists regarding nasogastric (NG) lavage in a patient with a GIB. The NG lavage is theorized to help differentiate an UGIB from a LGIB and aid in the timing of endoscopy. However, a systematic review found the NG lavage was a poor predictor of an UGIB. The sensitivity ranged from 42 to 84 % and the specificity ranged from 54 to 91 %. Most importantly, a negative NG lavage was unable to exclude an UGIB, and this is the main purpose of the procedure [8]. These disappointing results are not that surprising if one considers the limitations of the procedure. False-negative results are known to occur from bleeding duodenal ulcers because the blood may not be able to reflux across the pyloric sphincter. Physicians are also not accurate in detecting bile in the aspirate, which is critical for determining a truly negative lavage. False-positives can come from the trauma of insertion or the subjective interpretation of the aspirate. Is it positive if just a few bloody flecks are seen? Is it positive if it clears with 50 cc of saline? Simply put, the NG lavage cannot rule out an UGIB, and other clinical factors are superior to the NG lavage to confirm or exclude an UGIB.
The second theorized benefit of the NG lavage is to determine who may benefit from early endoscopy. Endoscopy is currently recommended within 24 h for patients with an UGIB. However, very little is known about which patients, if any, would benefit from an urgent endoscopy (within 12 h). Conceptually, patients with bloody NG lavages may have high-risk lesions that could benefit from urgent endoscopy. However, many studies have not found any benefit using the NG lavage to select patients for an urgent endoscopy [2, 9].
Additionally, the NG lavage has been described as the most painful emergency department procedure and is more painful than a lumbar puncture or fracture reduction [10]. NG tubes also have rare complications that include pulmonary malposition, pneumothorax, respiratory failure, perforation, vocal cord injury, pneumonia, epistaxis and death.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







