INTRODUCTION AND EPIDEMIOLOGY
This chapter discusses the features of low-probability or possible acute coronary syndrome (ACS), which includes patients who have chest pain or another equivalent ischemic symptom but no objective evidence of acute coronary ischemia or infarction—that is, no characteristic ECG ST-segment elevation or depression and normal levels of cardiac markers. Patients with diagnostic ECG or cardiac marker levels or with other high-risk features are discussed in chapter 49, “Acute Coronary Syndromes.”
Of ED patients with undifferentiated chest pain, 7% will have ECG findings consistent with acute ischemia or infarction, and 6% to 10% of those in whom cardiac markers are ordered will have initially positive results.1 The remaining patients who do not have diagnostic ECG changes or initially positive cardiac marker results have low-probability or possible ACS. The evaluation of those with possible or actual ACS costs approximately $10 billion to $12 billion each year in the United States.2
Of all patients with possible ACS, 5% to 15% ultimately prove to have ACS.3 The rate of discharge from the ED for patients with ACS remains approximately 4%. Patients with ACS who are discharged home from the ED have worse clinical outcomes and higher mortality compared with patients who are initially hospitalized. The clinical data readily available to the emergency physician, such as historical features, examination findings, and ECG results, cannot exclude ACS among most patients, because 3% to 6% of patients thought to have noncardiac chest pain or a clear-cut alternative diagnosis will have a short-term adverse cardiac event.4,5 Therefore, most patients with possible ACS undergo further observation and testing.
PATHOPHYSIOLOGY
ACS is a constellation of signs and symptoms resulting from an imbalance of myocardial oxygen supply and demand. There are three general ACS classifications: unstable angina, non–ST-segment elevation myocardial infarction (NSTEMI), and ST-segment elevation myocardial infarction (STEMI). Unstable angina is a type of ACS with no elevation of biomarkers and no pathologic ST-segment elevation, resulting in ischemia but not infarction. Acute myocardial infarction (AMI) occurs when myocardial tissue is devoid of oxygen and substrate for a sufficient period of time to cause myocyte death. NSTEMI is characterized by biomarker elevation and no pathologic ST-segment elevation. STEMI is characterized by ST-segment elevation and biomarker elevation (STEMI), although biomarker elevation is not required at onset to make this diagnosis. Detailed discussion is in chapter 49.
The distinction between NSTEMI and unstable angina is based on elevated cardiac markers of necrosis in the case of NSTEMI. Troponin I and troponin T are the most specific cardiac markers of cell injury or death available. These biomarkers may not reach detectable thresholds for up to 6 hours after infarction. Patients presenting soon after infarction may have normal biomarker results and initially be categorized as having possible ACS. Patients with evolving myocardial infarctions represent approximately 4% of patients undergoing serial cardiac markers and generally have other high-risk features of ACS such as ST-segment depression.6,7
CLINICAL FEATURES
The history is one tool to help assess patients with suspected or possible ACS but cannot be used to exclude ACS. Obtain detailed information, including symptom quality, location, duration, severity, associated symptoms, precipitating and relieving factors, and similarity to prior episodes. Consider other noncardiac but life-threatening causes of chest pain (see Tables 48-3 and 48-4 in chapter “Chest Pain”).
Among patients with possible ACS, historical features can be categorized as low risk, probable low risk, probable high risk, and high risk. However, even patients with low-risk features or in a low-risk category have a residual risk of ACS.8 Lowest risk features include pleuritic, positional, reproducible, or sharp/stabbing pain. Another low-risk feature is pain that is not exertional or located in a small inframammary area. Higher risk features include chest pressure (positive likelihood ratio [LR+] 1.3), pain similar to or worse than prior cardiac pain (LR+ 1.8), and associated nausea/vomiting or diaphoresis (LR+ 1.9 and 2.0, respectively). The highest risk features include radiation to the right arm or shoulder (LR+ 4.7), left arm (LR+ 2.3), or both arms or shoulders (LR+ 4.1), and exertional chest pain (LR+ 2.4).
Traditional cardiac risk factors, such as hypertension, diabetes mellitus, tobacco use, family history of coronary artery disease (CAD) at an early age, and hypercholesterolemia, are modestly predictive of the presence of CAD in asymptomatic patients. In acute decision making, cardiac risk factors are poor predictors of cardiac risk for myocardial infarction or other ACS.9 CAD is rare in patients <30 years old, but possible and usually accompanied by other risk(s).
Although a true alternative diagnosis decreases the likelihood of ACS, many patients with ACS are mistakenly diagnosed with gastric reflux or musculoskeletal pain.5 Clinical response to treatment with antacids (18% to 45% of ACS patients have relief of pain with antacids), viscous lidocaine, or nonsteroidal anti-inflammatory medications cannot exclude ACS. Lack of pain relief with nitroglycerin is similarly unreliable, because 65% of patients with an ACS in one study failed to have relief of pain.
Prior cardiac testing (previous ECG tracings, echocardiograms, cardiac catheterization reports, and stress testing reports) aids the ED evaluation. ECG changes offer strong bedside evidence of new disease. Recent cardiac catheterization reports are especially useful, because a truly negative (defined as no luminal irregularities) result is associated with a very low incidence of subsequent infarction or ACS in the next 2 years. Although a prior positive stress test increases the likelihood of a subsequent acute cardiac event,10 a prior negative stress test provides little reassurance that a current chest pain event is benign. Patients with a recent negative evaluation for ACS that included objective cardiac testing (mostly stress testing) have a 6-month incidence of ACS as high as 14%. Furthermore, patients with a prior negative stress test have a similar incidence of ACS compared with those with no prior stress testing when presenting with chest pain to the ED.11 Overall, previous stress test results add evidence but cannot confirm disease presence or absence.
Usually, the exam in the low-risk or possible ACS patient seeks complications or alternative causes of the symptoms. When an alternate diagnosis is not clear, the physical examination alone cannot distinguish between those with and without ACS. However, pay special attention to examination findings that make ACS more or less likely. High-risk features on physical examination are uncommon and include signs of cardiac failure such as hypotension, diaphoresis, pulmonary rales, jugular venous distention, new mitral regurgitation, bradycardia, tachycardia, and an S3 cardiac gallop.12 Cardiac rate abnormalities are also a high-risk feature, including either bradycardia (seen with ischemia, especially in the inferior myocardium, or infarction that has led to disturbance of the conduction system) or tachycardia (may signify pump failure, pain, or stress, or could be a clue to an alternative diagnosis such as pulmonary embolism). Positional changes in the pain severity can be easily assessed at the bedside and may suggest pericarditis or another pleural-based syndrome as a cause of pain.
The classic patient with possible ACS has chest pain or another symptom (weakness, dyspnea, other upper body pain), no clear alternative cause, and no clear evidence of cardiac injury or stress on ECG and biomarker tests. In this group, the likelihood of ACS is >2% but still low, making the diagnosis of possible ACS.
DIAGNOSIS
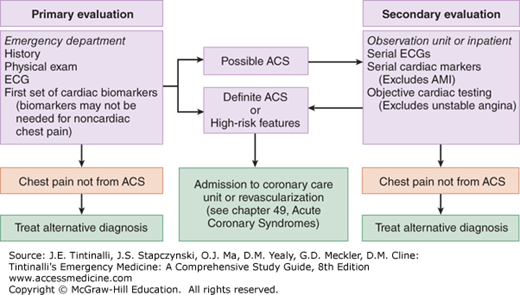
PRIMARY EVALUATION
The goal during the primary evaluation is to distinguish patients with definite ACS from those with possible ACS and those with symptoms that are definitely not from ACS. This process involves gathering information from the history, physical examination, ECG, chest radiography, and medical record. This information is used to produce an initial risk assessment that guides the subsequent laboratory testing and initial treatment.
For adult patients with chest pain in whom cardiac causes are possible, obtain a 12-lead ECG rapidly12,13 and compare to any previous tracings. Patients with ST-segment elevation, a new left bundle-branch block, or ST-segment depression have an ACS and are treated as outlined in chapter 49. Between 1% and 6% of patients with a normal ECG will ultimately prove to have NSTEMI, and at least another 4% will prove to have unstable angina.12 Obtaining follow-up ECG(s) in patients with ongoing or worsening symptoms helps detect changes diagnostic of ACS, or it can show reversal of previous ST-segment or T-wave changes thought to be old—both define dynamic changes and the high likelihood of ACS. In the setting of dynamic ECG changes, incidence of CAD is 84% with classic anginal symptoms and 8% with nonclassic anginal symptoms as assessed by coronary angiography.14
Chest radiography provides additional cardiovascular information, including cardiac silhouette size, pulmonary edema, and aortic contour. Additional causes of chest pain, such as pneumothorax, bony metastasis, rib fracture, and pneumonia, may also be detected on chest radiography. Patients presenting with anterior chest pain have findings on chest radiography that influence management 14% of the time.
The goal of early decision making is to determine if the patient actually belongs in the possible ACS cohort and is at high enough risk to warrant cardiac testing. All available data are used to create a composite picture for decision making. Some posit that when the pretest probability of ACS is ≤2%, further testing is not indicated15; others have suggested a threshold of <1% to stop testing.16 Detecting patients with a zero chance of any ACS in the presence of ACS-like symptoms is virtually impossible.
Most computer algorithms, risk scores, and clinical decision rules have been unsuccessful at identifying a very-low-risk (<2%) cohort that does not require further testing. The HEART score is one tool that identifies low-risk patients who are eligible for evaluation and possible early discharge home from the ED (Table 51-1). Large-scale validation data are lacking, although current evidence suggests that patients with a HEART score of 0 to 3 have a 1% to 2% risk for major adverse cardiac events within 6 weeks of presentation.17,18
| Points | |
|---|---|
History
| 2 1 0 |
ECG
| 2 1 0 |
Age
| 2 1 0 |
Risk factors*
| 2 1 0 |
Troponin
| 2 1 0 |
| Total |
The Heart Pathway involves using the HEART score with the addition of a 4- to 6-hour repeat troponin to increase the sensitivity for major adverse cardiac events.19 Preliminary data note 100% sensitivity for major adverse cardiac events while decreasing the amount of index visit cardiac testing (CT or stress imaging).
The Accelerated Diagnostic Protocol uses Thrombolysis in Acute Myocardial Infarction (TIMI) study20 risk scores and ED testing to stratify risk (Table 51-2). Patients with ACS-consistent pain, a Thrombolysis in Acute Myocardial Infarction score of 0, no new ischemic changes on ECG, and negative troponins at 0 and 2 hours have a very low risk of subsequent cardiac events and may be discharged. In a prospective observational study, the Accelerated Diagnostic Protocol was 99.7% sensitive (95% confidence interval [CI] 98.1% to 99.9%) for major adverse cardiac events at 30 days.21 A subsequent randomized trial demonstrated that 19.3% of patients were discharged within 6 hours using the Accelerated Diagnostic Protocol compared to 11.0% of control group patients (P = 0.008), and 1.9% (95% CI 0% to 10%) of patients had a major adverse cardiac event in 30 days.22 This method also needs wider impact testing to determine safety and utility before mainstream implementation.
When risk-stratifying patients with possible ACS, be wary of overconfidence in an alternative diagnosis, because the rate of ACS is as high as 4% among patients with chest pain and a “clear-cut” noncardiac alternative diagnosis.5 Reserve determinations of noncardiac chest pain to patients with a very low likelihood of coronary disease (<1%) and clear evidence of an alternative diagnosis or with atypical historical features. The rest of patients with ACS-consistent symptoms should have further testing.
After determination that the patient is appropriately categorized as possible ACS, further stratification occurs (Table 51-3).12,13,23 Patients in category I (AMI) and category II (probable acute ischemia) are discussed in chapter 49. Patients in category III (possible ischemia) and IV (probably not ischemia or stable angina pectoris) undergo primary and secondary assessments as detailed below.
| I. Acute myocardial infarction: immediate revascularization candidate |
II. Probable acute ischemia: high risk for adverse events (any of the following):
|
III. Possible acute ischemia: intermediate risk for adverse events. History suggestive of ischemia with absence of high-risk features, and any of the following:
|
IV. Possible acute ischemia: low risk for adverse events. Requires all of the following:
|
V. Definitely not ischemia: very low risk for adverse events. Requires all of the following:
|
Laboratory testing during the primary evaluation focuses on two goals: detecting myocardial cellular necrosis and excluding alternative causes of chest pain. Usual testing includes a CBC, serum electrolytes with renal function, and serum cardiac markers. Other testing is guided by the history and physical examination.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree