Surgical latex gloves have been worn for more than a century. Although numerous variations of the surgical glove were developed, Dr. William Halsted of Johns Hopkins Hospitals is often credited with their introduction. Before the adoption of routine surgical glove use, the surgical team members would disinfect their hands with carbolic acid, introduced by Dr. Joseph Lister, which is quite caustic. Dr. Halsted’s scrub nurse, Carolyn Hampton, was on the verge of quitting her job because she could no longer tolerate the irritation to her skin. In trying to resolve this issue, Dr. Halsted contacted the Goodyear Rubber Company in Akron, Ohio, which had just refined the rubber vulcanization process. In response to Dr. Halsted’s request, they manufactured rubber gloves for Ms. Hampton to wear, protecting her hands. Thus the first surgical gloves were developed to protect the hands of the worker, not to protect the patient.
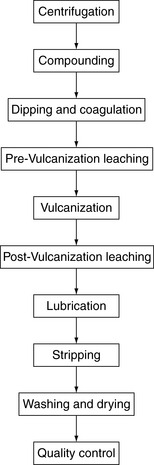
When Goodyear made the first rubber surgical gloves, they probably contained only natural rubber cross-linked with a sulfur compound. In addition, they most likely took many hours to cure and probably were expensive compared with today’s glove prices. For decades, medical gloves were so expensive that they were washed, checked for holes, powdered with talc, and sterilized repeatedly until they could no longer be used. Many nurses spent much of their time reprocessing surgical gloves.
As production methods improved, mass production of medical gloves reduced the costs of the gloves. In the 1960s single-use, disposable medical gloves were introduced. Low-cost medical gloves offered the convenience of throwing them away after use rather than rewashing and reprocessing them, which included steam sterilization. With the change to single-use gloves, the method for sterilization also changed to gamma irradiation. As a result, gloves were no longer washed or subjected to steam under pressure. The allergenic proteins in the gloves are water soluble. The proteins are denatured to some degree by steam under pressure. By removing both of these processes of steam and washing, the level of allergenic proteins in the gloves may have inadvertently increased, thus exposing wearers to greater levels of allergens. There is evidence of this in third world countries that still follow this practice. One study showed that in Venezuela, where gloves and catheters are reused, the incidence of latex allergy in the spina bifida population is not significantly higher than in the general population.
Allergic reaction to latex gloves was first reported in the literature by Downing in 1933. Reports of delayed allergic reactions appeared over the next several decades. NRL-induced anaphylaxis was first described in the literature in 1979, with a significant increase in the number of NRL-induced allergic reactions in the late 1980s. A number of events caused this rapid increase.
In the early 1980s the first cases of a new disease were being reported. We now know these were the first reports of human immunodeficiency virus (HIV) infection or acquired immunodeficiency syndrome (AIDS). The disease initially appeared to be found in the homosexual population but was soon found in persons with hemophilia and in infants whose mothers were infected with AIDS. In 1983 the Centers for Disease Control and Prevention (CDC) warned blood banks of a possible problem with the blood supply. Universal precautions were introduced in directives and guidelines issued by the CDC in 1987 and in standards published by the Occupational Safety and Health Administration (OSHA) in 1991 to protect HCWs and others from exposure to potentially contaminated blood and other bodily fluids. One of the key components of universal precautions is the use of gloves. One result of universal precautions was that HCWs who had historically not worn gloves (e.g., phlebotomists and dentists) began to wear gloves, and others who had sometimes previously worn gloves (e.g., nurses and physicians) began wearing them more frequently. It is estimated that glove use grew from 1.4 billion gloves in 1988 to 8.3 billion gloves in 1993. This resulted in increased exposure to both NRL and the chemicals used in the glove manufacturing process.
Universal precautions also resulted in the “Great Glove Shortage” of the 1980s, which had a significant impact on the manufacturing of latex gloves and other products. Scientists are not absolutely certain, however, whether changes in the manufacturing of latex gloves may have resulted in the increase in latex allergies. Manufacturers were having a difficult time keeping pace with the demand for surgical and examination gloves. In response to the demand for more raw latex to meet the shortage, plantation owners who grew and harvested the latex from their trees may have collected the latex before it had ripened. (Latex trees are not usually harvested until the plant is up to 7 years old, because the latex is considered immature.) This unripened or “green” latex contains proteins that are more allergenic or “potent.” In addition, because of the increased demand for latex, the manufacturers, who had been harvesting and storing the raw latex until it was needed, were using it as quickly as possible to make gloves. This decreased the storage time of the raw materials, which previously had helped to destroy allergens. This action removed a natural ripening time in the latex manufacturing process, again allowing latex with more allergenic proteins to be used in glove manufacturing.
Up until this time a number of glove companies had manufacturing facilities located in North America. The raw latex was shipped in large tankers to plants for glove production. Some of these companies relocated their manufacturing plants to Southeast Asia, closer to the plantations where the latex is harvested. In doing so, once again a natural ripening time was removed from the process.
At the same time, new manufacturers were entering the market to fill the gap between supply and demand. The majority of latex in the world is produced in Malaysia. According to the Malaysian Industrial Development Association (MIDA), over 400 new licenses were issued to manufacturers between 1985 and 1990. Some of these new manufacturers may not have had the requisite knowledge, skill, or equipment to make a high-quality glove, thereby bringing potentially inferior products to the market. As health care workers from the time can attest, there were gloves coming into the hospitals that would tear apart on donning or completely stick together and be unwearable. In contrast, by 1995 MIDA reported the total number of glove manufacturers had fallen to approximately 100.
Another potential contributing factor worth considering is the weather. Some have speculated that variables such as hybridization and seasonal variation played a role. A drought occurred in areas where latex manufacturing facilities were located, which may have caused the manufacturers to reduce the amount of washing or “leaching” of the gloves and other latex products, resulting in more high-protein gloves entering the market.
The implementation of universal precautions coincided with another significant event. It was reported that over a dozen people in the United States were killed related to the use of barium enema tips (MedWatch, 1996). The latex cuff of the barium enema tips was suspected of containing an extraordinarily high level of latex proteins, which was attributed to a manufacturing anomaly. The fact that latex proteins could provoke anaphylactic responses, potentially injuring and killing people, stunned health care facilities and the medical latex glove industry. At the same time, because of the tremendous increase in medical glove use and the high latex protein content of some latex gloves, reported latex allergies among HCWs began increasing (MedWatch, 1996).
By 1992 the U.S. Food and Drug Administration (FDA) convened a meeting in Baltimore of government, academia, manufacturers, and scientists to discuss latex allergy. By this time it was well known that the allergenic proteins in the latex were the culprit. This meeting led to FDA selection of the Lowry testing method for total proteins in gloves and subsequent FDA labeling requirements for “low protein” claims.
From 1992 to the present, the levels of extractable latex proteins and antigens and powder and rubber chemicals have declined in most medical gloves. Most examination gloves used in the United States today are powder-free and nonlatex. Use of nonlatex gloves is growing quickly with the advent of better gloves made of chloroprene and polyisoprene. However, approximately 35% of surgical gloves used in the United States today are still powdered. Glove powder has been implicated in the transfer of latex proteins in the health care environment.
Today the number of new latex allergy cases appears to be declining, likely as a result of using fewer powdered, high-NRL antigen gloves (Early, 2005). However, type IV reactions to gloves (e.g., chemical reactions—primarily caused by accelerators) continue to occur. The chemical sensitivities may appear to be on the increase just because there are fewer new latex allergy cases.