42 Key Issues in Pediatric Neurointensive Care
In this chapter we outline the epidemiology, presentation, course, and management of key disorders in pediatric neurointensive care. Critically ill infants and children with a compromised central nervous system (CNS) are complex patients and are often highly vulnerable to secondary brain injury. Minimizing physiologic derangements and optimizing therapy are essential from the scene through the pediatric intensive care unit (ICU). In most cases, transport to a specialized pediatric facility is desirable. Trained specialists in pediatric critical care medicine, pediatric neurologic surgery, and child neurology should deliver the ICU care to these infants and children, with appropriate pediatric ancillary support. The information provided in this chapter is germane to practitioners involved in stabilization, emergency treatment, and transport, and to pediatric subspecialists at the tertiary care centers. Recommendations in the areas of pediatric trauma (head and spinal cord injury), procedures, and monitoring are addressed in Chapters 30, W24 (Pediatric Intensive Care Procedures), and 210. Neurointensive care issues relevant to the field of neonatology are outside the scope of this chapter; specialized textbooks and/or reviews in this area should be sought for information in that field.
 Issues Unique to Pediatrics
Issues Unique to Pediatrics
Central nervous system Insults in Infants and Children
Unlike in adults, atherosclerotic vascular disease resulting in stroke, intracerebral hemorrhage, and cardiopulmonary arrest plays little role in pediatric neurointensive care. For example, cardiopulmonary arrest in infants and children results primarily from asphyxia rather than myocardial infarction. Similarly, traumatic brain injury (TBI) in infants younger than 2 years of age is largely the result of abusive head trauma (shaken baby syndrome, child abuse). Unique issues in victims of child abuse, such as chronic injury or delay in presentation, contribute to important differences in diagnosis, treatment, and outcome. The specific CNS insults relevant to pediatric neurointensive care include TBI and spinal cord injury, cardiopulmonary arrest, status epilepticus, stroke, critical CNS infections, postoperative neurosurgical conditions, and several other less common disorders; traumatic brain and spinal cord injury are addressed in Chapters 38 and 39.
Age-Related Differences in the Response to Central nervous system Insults
Brain Water and Blood-Brain Barrier
Many biochemical, physiologic, and physical factors exhibit large fluctuations during brain development. Although the magnitude of these changes are most dramatic during prenatal development, they may contribute to age-related differences in response to critical CNS disorders.1,2 Large decreases in brain water content occur during postnatal development into adult life.3–5 These changes are global and correlate with the amount of myelination. The impact of these changes on edema formation after brain injury is unclear; however, the rapid and diffuse cerebral swelling phenomenon described in many CNS insults in infants and children may be related to this high water content in the immature brain. This is suggested by studies showing that parenchymal injection of glutamate into the immature (but not adult) rat brain rapidly produces a large area of edema.6 The rapidity of development and the great magnitude of edema may result in part from rapid diffusion of glutamate and other mediators through the immature brain. In contrast to the changes in brain water during development, there is little evidence to support similar changes in blood-brain barrier permeability.7,8 However, studies in experimental models suggest that the immature blood-brain barrier is highly vulnerable to injury.9–11 Blood-brain barrier permeability after CNS insults has received little study in pediatric patients.
Cerebral Blood Flow and Energy Metabolism
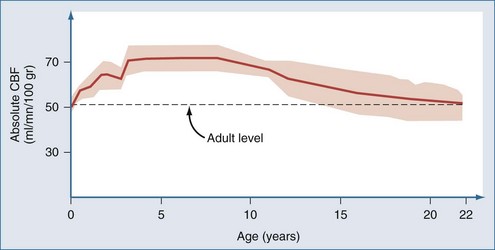
Postnatal changes in cerebral blood flow (CBF) and energy metabolism have been reported in numerous mammalian species including humans.12–19 In all cases, CBF is quite low both before birth and during infancy, rapidly increases to a peak during childhood, and then decreases to a plateau with a gradual decline with increasing age during adulthood. In a study of 42 normal infants and children, cortical CBF in newborns was between 30 and 45 mL/100 g/min—lower than that reported in adults. In contrast, cortical flow in children between the ages of 5 and 6 years was between 50% and 85% higher than in adults. CBF decreased to adult values by about age 15 years (Figure 42-1).20,21 Increased CBF in children (versus either adults or infants) corresponds to the period of maximal postnatal “brain growth,” specifically, maximal increases in the number of synapses.22–24 Similarly, cerebral metabolic rate for glucose is maximal in children between the ages of 3 and 9 years.17 The impact of these factors in CNS injury is poorly understood. Hyperemia after injury has been implicated as an important facet of the pathophysiology of pediatric CNS injury. Because the level of CBF in the normal child is greater than in adults, the frequency of hyperemia in children is probably lower than has been suggested. Hyperemia in most gray matter structures in children between the ages of 3 and 10 years should probably be based on a flow value greater than about 70 mL/100 g/min19–2124 rather than the value of about 45 mL/100 g/min suggested for adults.25 Alterations in metabolic demands after injury must also be considered.
Cerebral Perfusion Pressure
Cerebral perfusion pressure (CPP; mean arterial blood pressure–intracranial pressure [ICP]) is a critical determinant of CBF outside the limits of autoregulation or when autoregulation of blood pressure (BP) is disturbed. In adults, the normal range for CPP is generally accepted to be between 60 and 150 mm Hg.26,27 Based on studies in normal immature animals, the lower limit for BP autoregulation of CBF is directly related to age.28–30 This is anticipated, since CPP is a function of arterial blood pressure, which is dependent on age. Unfortunately, few data are available on normal values for CPP in infants and children. A mean value of 37.5 ± 4.9 mm Hg (±SD) was reported in normal preterm infants.31 The lower limit of BP autoregulation was not determined. There are also limited data available on the lower limit of BP autoregulation of CBF in brain-injured infants and children. A study carried out in 1983 in 17 infants and children with meningitis and encephalitis showed a critical threshold for CPP of about 30 mm Hg.32 However, survival, not CBF, was the outcome variable in that study. Muizelaar and coworkers20,21 and Sharples and colleagues33 examined CBF autoregulation after TBI in children; however, values for BP autoregulation of CBF for infants and children were not determined. Recently, Vavilala et al.34 studied CBF autoregulation in 53 healthy infants and children. Surprisingly, the lower limit of autoregulation was between 50 and 60 mm Hg across the age groups of younger than 2 years, 2 to 5 years, 6 to 9 years, and 10 to 14 years. This important study suggests that there is substantially less autoregulatory reserve (the difference between baseline MAP and the lower limit of autoregulation) in infants and young children than in older children or adults. It would suggest that modest BP reductions in infants with severe TBI could compromise CBF. This may help explain the important deleterious effects of hypotension as a side effect in recent RCTs in pediatric TBI.35 It also suggests that the lower limit for CPP in brain injured infants and young children of ~50 mm Hg might be wise. Two recent studies also suggest that the presence of mild hypertension after severe TBI is associated with improved outcome in infants and children.36,37 However, the impact of inducing mild hypertension in this setting on outcome remains to be studied.
Myelination
In humans, considerable myelination occurs during postnatal life.23 The impact of this process on the age-related response in pediatric CNS injury is not known but has been suggested by many to contribute to enhanced plasticity in the pediatric brain.
Excitotoxicity
Increases in brain interstitial concentrations of excitatory amino acids such as glutamate are part of a fundamental response to CNS insults across all ages.38–47 Excitotoxicity-mediated damage after brain injury has been reported in laboratory models in mature and immature animals and is suggested in clinical reports in children.39,43–47 There are, however, important age-dependent facets of excitotoxicity. At several periods in development, large numbers of excitatory amino acid receptors are produced, and these periods correlate temporally with increased synaptic plasticity.40–43 Experimental data strongly suggest that the immature brain is at great risk for excitotoxicity.39–41 In hypoxia-ischemia models, studies in immature animals (particularly those modeling the newborn) suggest that glutamate receptor antagonists such as MK-801 are potent neuroprotectants.40,41,43 The results of clinical trials in adults of agents targeting this receptor may not predict their effectiveness in infants or children with critical CNS insults. Further study in children is warranted.
Apoptosis
Experimental models and human data have made it increasingly clear that cells dying after CNS insults can be categorized on a morphologic continuum from necrosis to apoptosis.48–50 The event involved in the cascades of neuronal death after CNS insults is discussed in detail in Chapter 29. The importance of balanced apoptosis (or programmed cell death) in embryogenesis and recent reports examining apoptosis in experimental TBI suggest that there may be important age-related differences in the cell death cascades in response to traumatic or ischemic brain injury.51 For example, neurons in developing animals appear to be more vulnerable to apoptosis than in mature animals.48,51 There are also data supporting the concept that physiologic levels of excitatory amino acids are necessary for neuronal survival in the developing brain.52 The implications on these data in experimental animals must be assessed with caution, but they raise concern about the ability of therapies such as barbiturates or inhibitors of excitatory amino acid receptors to actually induce neuronal death during development. The fetal alcohol syndrome is the prototypical condition cited in this regard.53 What remains unclear, however, is if this enhanced apoptotic response to CNS injury is limited to prenatal development or if it is important during treatment of infants and children in the pediatric ICU. Nevertheless, an important role for apoptosis in pediatric brain injury is suggested by the fact that analysis of cerebrospinal fluid (CSF) in infants and children with severe TBI has provided some of the most compelling molecular data for the participation of these pathways in humans.2 These data include participation of death effectors such as cytochrome-c and Fas receptor/ligand interactions and failure of antiapoptotic pathways in infants and children with poor outcome after severe brain injury.2,54–57 How these findings will influence our therapies remains to be determined, but they suggest that apoptotic neuronal death may represent a particularly important therapeutic target in pediatric neurointensive care.
Extracerebral Factors
Hypotension and hypoxemia are the two most important secondary insults in patients with critical CNS disorders. Hypotension is the most important extracerebral factor associated with poor outcome after severe TBI.58 This may contribute to the high mortality rate (62%) in this condition in children younger than age 4 years.59 Nearly 50% of these children present with shock, versus only 30% of adults.59 The limited blood volume of infants and young children make relatively small amounts of blood loss from scalp lacerations or other foci important. In contrast, the immature brain and cardiovascular systems are resistant to hypoxic-ischemic insults compared with mature individuals.60 The duration of asphyxia resulting in cardiac arrest is inversely related to age.61–64 Resistance to asphyxia-induced cardiac arrest in the immature individual, however, could have complex effects. For example, children may survive protracted episodes of hypoxemia and hypotension that would be lethal in adults. Resistance of the immature myocardium to asphyxia does not preclude the development of cerebral damage from hypoxemia, because between 25% and 56% of children who suffer asphyxia without cardiac arrest have poor neurologic outcome.65 This might also explain some of the severe pathology seen in infants after abusive head trauma, in which apnea, seizures, and agonal states occur.66 Recently Ichord et al.67 showed that a hypoxic-ischemic injury pattern was commonly seen on diffusion-weighted magnetic resonance imaging (MRI) in victims of abusive head trauma. Similarly, Berger et al.68 showed that the serum biomarker profile of neuron-specific enolase in infants with abusive head trauma was more similar to that seen in children with asphyxia than TBI.
Unlike adults, atherosclerotic vascular changes are largely absent in children. This influences pathophysiology. Although normal aging produces a gradual decline in CBF, this decline is accentuated in adults by the presence of risk factors for stroke (e.g., diabetes, cigarette smoking, hypertension), which enhance incipient cerebrovascular disease.69 Atherosclerosis also limits the ability of cerebral circulation to respond to a metabolic challenge.70–73 Some adults may even have maximally dilated cerebral vessels in the resting state. The potential of these factors to unfavorably affect outcome in adults (versus children) is obvious. Ethanol consumption is associated with severe TBI in adults, with as high as 50% of patients having positive blood alcohol levels.74–77 Chronic and acute alcohol consumption can have either detrimental or beneficial effects on brain injury.77 Ethanol use or intoxication is uncommon in pediatric TBI, particularly in infants and young children.
 Specific Diseases or Conditions
Specific Diseases or Conditions
Cardiopulmonary Arrest
Cardiopulmonary arrest in adults is addressed in detail in Chapter 33. Although some of that chapter is germane to pediatric patients, the importance of asphyxia as the etiology in children mandates a separate discussion.
Epidemiology
The causes of cardiopulmonary arrest in childhood are heterogeneous. Causes of arrest in the prehospital setting include trauma, sudden infant death syndrome, poisoning, and respiratory distress secondary to drowning, choking, severe asthma, or pneumonia.78 Traumatic arrest secondary to exsanguination, massive head injury, or airway compromise is the leading cause of death in childhood and young adulthood. Nontraumatic arrest typically occurs as a consequence of hypoxemia and hypercarbia, leading to respiratory arrest, bradycardia, and ultimately asystole or pulseless electrical activity.78–80 Ventricular tachycardia or fibrillation occurs less commonly in children than adults, but it is not rare; 5% to 15% of children with prehospital arrest have these rhythms.81–83 The majority of arrests in the prehospital setting occur in previously healthy patients, whereas most in-hospital arrests occur in children with preexisting medical conditions.84 Children with special healthcare needs are especially vulnerable to acute deterioration.
Outcome
The rate of survival from pediatric cardiopulmonary arrest is about 13%, with survival from in-hospital arrest greater than that from prehospital arrest (24% versus 9%).80 Asystolic patients have the lowest rate of survival (~5%), whereas patients with ventricular fibrillation or ventricular tachycardia have higher rates of survival (~30%). Patients presenting with isolated respiratory arrest have the highest rate of survival (~75%).85,86 Witnessed arrest and bystander cardiopulmonary resuscitation (CPR) are associated with survival, whereas CPR of greater than 30 minutes and administration of more than two doses of epinephrine are associated with poor outcome.78,81,87,88 About 60% of survivors will have good neurologic outcome, with the remainder showing severe disabilities. Intermediate outcomes are uncommon. Reported mortality rates for children remaining comatose after brain injury range between 34% and 73% dependent on whether TBI is included.89–94 Accurate prediction of poor outcome in this group can enable withdrawal of support and decrease the possibility of “rescuing” children to survival in a neurologically devastated state.95,96 Predictors of poor outcome in children include remaining comatose at 24 hours, a Glasgow Coma Scale (GCS) score of less than 5, absence of spontaneous respirations, absence of pupillary reflex, and specific abnormalities found on electroencephalography (EEG) or after testing of somatosensory evoked potentials. Predictors of poor outcome should be applied with caution to children suffering cardiopulmonary arrest caused by drug overdose or hypothermic exposure (ice-cold water drowning) in which good outcomes have been reported in some cases after even prolonged durations of arrest.
Treatment
If cardiopulmonary arrest occurs, the most important first step is to provide immediate CPR. Many infants and children, especially in the prehospital setting, will be rescued solely by the administration of CPR.78 Important differences are emerging in resuscitation of adults versus children with cardiac arrest. Although there has been a general movement toward bystander compression-only CPR in adults, recently Kitamura et al.97 compared conventional versus compression-only CPR in over 5000 children in Japan. In arrests of noncardiac origin, both survival and favorable neurologic outcomes were better in children given conventional CPR. In addition, outcomes were similar in the setting of arrests of cardiac origin. This study strongly suggests that the lay public should be taught conventional CPR for all children who suffer cardiac arrest. In addition the technique for compressions in children is different than in adults. Only one hand is used to deliver chest compressions to children younger than age 8 years. Two methods are approved for delivering chest compressions to infants. When two or more rescuers are available, one rescuer provides chest compressions by encircling the chest with two hands and depressing the sternum with both thumbs while the other rescuer provides ventilation (Figure 42-2). When only one rescuer is present, two fingers from one hand are used to provide chest compressions and the other hand is used to maintain the head-tilt. Providing adequate ventilation is especially important for children, because most pediatric arrests are secondary to airway compromise. In contrast, adults frequently suffer from cardiac causes of arrest and require intensified efforts at providing chest compressions and early defibrillation. Thus, the recommended ratio of chest compressions to ventilations for young children is 5 : 1, compared with a ratio of 15 : 2 for older children and adults. Once the patient is intubated, ventilations should be asynchronous. Although ventricular fibrillation and ventricular tachycardia are uncommon in children, survival with this rhythm is high (about 30%), so cardiac rhythm should be ascertained as early as possible.80 Automated external defibrillators that can deliver a 50-J dose are now available and are appropriate for use in children aged 1 to 8 years.98
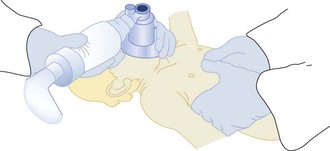
Figure 42-2 Two-person technique for cardiopulmonary resuscitation in infants and young children.
(Reprinted from Pediatric Basic Life Support. Guidelines 2000 for Cardiopulmonary and Emergency Cardiovascular Care: International Consensus on Science. Circulation 2000;102(Suppl):I253–90.)
Intubation of pediatric patients is a difficult task for inexperienced providers. Furthermore, the short length of the trachea combined with patient movement during transport and patient care can easily result in displacement of the endotracheal tube.99 Secondary confirmation of tracheal tube placement is critical. End-tidal CO2 detection is the method most commonly utilized for secondary confirmation of endotracheal tube placement in children. However, a false-negative reading can occur when circulatory collapse is so severe that CO2 is not delivered to the alveolar space. If CO2 is not detected during CPR, tube placement can be confirmed by visualizing the airway with a laryngoscope. Although no single confirmation technique is 100% reliable in all circumstances, some effort of secondary confirmation of tube placement can be helpful.
Patients are initially resuscitated using 100% oxygen. The rationale is that hypoxia often causes or contributes to the development of cardiac arrest, and an oxygen debt accumulates during cardiac arrest. However, there is increasing awareness that oxygen might contribute to reperfusion injury, and thus prolonged delivery of unnecessarily high concentrations of oxygen should be avoided.100,101
Adults resuscitated from cardiac arrest demonstrate intact cerebrovascular reactivity with evidence of hyperventilation-associated ischemia.102 Although there is evidence that injured brain has diminished metabolism, which may offset the decrease in blood flow, it seems prudent to avoid decreasing CBF to injured brain. Therefore, hyperventilation should be reserved for patients with signs of cerebral herniation syndrome or suspected pulmonary hypertension. In addition to avoiding purposeful hyperventilation, it is prudent to guard against inadvertent hyperventilation during patient transport.103 Increased use of quantitative continuous CO2 monitors throughout the health care system would decrease the occurrence of inadvertent hyperventilation.
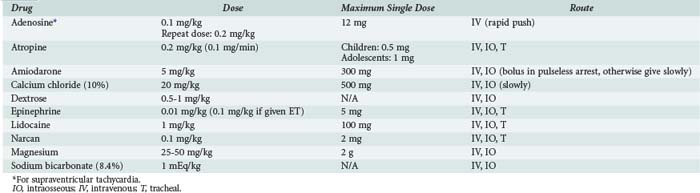
The most commonly used drugs in pediatric resuscitation are epinephrine, atropine, and sodium bicarbonate (Table 42-1). Magnesium and calcium are reserved for specific indications such as torsades de pointes, hypocalcemia, and calcium channel blockade. Amiodarone has recently been added to the American Heart Association (AHA) pediatric algorithms, based on extrapolation from adult experience.104 Adults with ventricular fibrillation or ventricular tachycardia in the prehospital setting are more likely to be successfully defibrillated after IV administration of amiodarone compared with lidocaine.105 Accordingly, amiodarone (5 mg/kg bolus) is a therapeutic option for children with pulseless arrest. Amiodarone (5 mg/kg infused over 20 to 60 min) is also an option for ventricular tachycardia with a pulse but should be used with extreme caution because of the risk for profound hypotension. Vasopressin has been added to the AHA adult algorithms as an alternative to epinephrine on the basis of its improved myocardial and CBF effects. However, subsequent clinical data in adults have not consistently yielded positive results, and pediatric data are limited to small case series.106,107 The optimal vasopressor for hemodynamic support after return of circulation in children is not known.
Extracorporeal membrane oxygenation (ECMO) has been used to successfully resuscitate children from selected causes of in-hospital cardiac arrest.108–112 ECMO-CPR provides greater cerebral and myocardial blood flow than either closed- or open-chest CPR and facilitates titration of temperature, blood flow, and oxygen-carrying capacity. Good outcomes have been documented with the use of ECMO even when initiated after durations of conventional CPR typically associated with poor outcome. It is best reserved for patients with reversible conditions or as a bridge to cardiac transplantation.
Post-Resuscitative Care
Temperature control is a priority for patients who remain comatose after cardiac arrest. Adults cooled to 32°C to 34°C for 12 to 24 hours after resuscitation from ventricular fibrillation demonstrate improved survival and neurologic outcome.113,114 In contrast, fever worsens outcome in experimental models of brain injury and has been associated with worse clinical outcome in adults with ischemic brain injury. Children resuscitated from cardiac arrest often develop mild hypothermia followed by delayed fever.115 There is a consensus that initial hypothermia, if tolerated, should be permitted to continue and fever should be vigilantly avoided. The practice of inducing hypothermia in normothermic children is more controversial. Experimental models using either pediatric mechanisms of injury (asphyxia, hypovolemic shock) or examining the immature brain suggest a beneficial effect of induced hypothermia. However, clinical data are limited and there is a concern about hypothermia-impaired immune function and risk of pneumonia/sepsis.116,117 Clinical trials of induced hypothermia for neonatal asphyxia have been remarkably positive,118–120 and important data in newborns with asphyxia indicate that even one degree of hyperthermia after the insult is associated with neurologic morbidity.121 This supports the need for targeted temperature management after cardiac arrest.
Sustained elevation of ICP may be more common after asphyxial arrests versus arrests of cardiac origin122 and is a poor prognostic sign in children with drowning. ICP monitoring fell out of favor in the 1980s when it was found to not influence outcome in small case series.123 However, studies using contemporary ICP-directed therapy (perhaps including induced hypothermia) deserve reevaluation.
Miscellaneous
Most pediatric victims of cardiopulmonary arrest will not be successfully resuscitated. The difficulty of accepting this reality often results in prolonged attempts at resuscitation. The AHA guidelines state, “In the absence of recurring or refractory ventricular fibrillation or ventricular tachycardia, history of a toxic drug exposure, or a primary hypothermic insult, resuscitative efforts may be discontinued if there is no return of spontaneous circulation despite advanced life support. In general, this requires no more than 30 minutes.”104 This acknowledges the futility of prolonged resuscitative efforts and empowers clinicians to feel permitted to stop resuscitative efforts. The guideline does not mandate stopping at a specific duration of CPR, but clinicians should recognize that the chance of survival with lifelong severe disabilities correlates with the duration of CPR.
Surveys indicate that most family members would like to be present during resuscitation attempts of a loved one124–127; presence during resuscitation can help family members adjust to the death.128,129 Although allowing family presence during resuscitation requires planning and additional resources, when done properly it is worth the effort. Perhaps one of the most disheartening statistics in resuscitation research is the high divorce rate (up to 90%) of parents after the death of a child. Thus, pastoral and social services can be integral components of care during both the acute resuscitation event and long-term follow-up.
Status Epilepticus
Status epilepticus is a pediatric emergency traditionally defined as either a continuous seizure of at least 30 minutes or more than two discrete seizures without complete recovery of consciousness. Refractory status epilepticus is defined as failure of two first-line antiepileptic medications to treat this condition for greater than 60 minutes. Many children with refractory status epilepticus have new or established CNS lesions.130
Epidemiology and Etiology
The incidence of pediatric status epilepticus from a prospective study is 40 cases/100,000 per year. Infants younger than 1 year of age have the highest incidence at 150 cases/100,000 per year.131 More than 90% of cases are convulsive status epilepticus. The first episode of status epilepticus occurs at a mean age of 4.2 years.132 There is a slight male predominance in status epilepticus.131,133
There are five etiologic categories of status epilepticus that have bearing on treatment and prognosis. A child with idiopathic or cryptogenic status epilepticus has no prior history of seizures and no known risk factors. Atypical febrile status epilepticus occurs during fever in children with no prior history of seizures without fever. Children with acute symptomatic status epilepticus have new CNS lesions such as encephalitis, trauma, tumor, stroke, or anoxia. Children with remote symptomatic status epilepticus have preexisting CNS lesions and therefore a lowered seizure threshold. In these children, status epilepticus can occur without provocation, sometimes even years after the initial insult. Finally some children have status epilepticus resulting from progressive encephalopathy, including neurodegenerative diseases, malignancies, and neurocutaneous syndromes (Box 42-1).131,133,134
In one study, status epilepticus accounted for 1.6% of total pediatric ICU admissions, and etiology varied with age. In children younger than 2 years of age, acute symptomatic status epilepticus from meningitis and encephalitis accounted for 51% of cases, whereas remote symptomatic status epilepticus in children with a prior diagnosis of epilepsy was seen in 16% of children. Older children were more likely than younger children to have a history of epilepsy.133 Mortality rates for status epilepticus in children are between 3% and 6%.131,134 Mortality is dependent on etiology, age, and duration of status epilepticus. Mortality rates of 0% and 12.5% were seen when patients were divided into either unprovoked/febrile status epilepticus or acute CNS insult/progressive encephalopathy groups, respectively.133 Morbidity risk varies from between 11% and 25%. Infants are at great risk for morbidity because the etiology in this group is commonly acute symptomatic status epilepticus. Neurologic sequelae of status epilepticus include epilepsy, recurrence, mental retardation, and motor disorders. However, many of the morbidities can be attributed to the underlying disease and not status epilepticus per se. Risk of recurrence in the category of idiopathic status epilepticus is less than 5%. In contrast, recurrence of status epilepticus in children in the acute symptomatic groups can be as high as 60%.131,135 Systemic complications occur with increasing frequency in proportion to the duration of status epilepticus, the most important being respiratory failure and cardiovascular compromise and autonomic and metabolic disturbances.136
Diagnosis
Status epilepticus can be convulsive or nonconvulsive when comparing clinical events with electrographic information. Convulsive seizures either begin as generalized seizures or progress from partial seizures. Nonconvulsive seizures are characterized as having subtle clinical signs such as nystagmus, irregular clonic twitches along with decreased consciousness, and/or ictal discharges on EEG. Included under the subheading of nonconvulsive seizures are complex and simple partial and absence seizures.137
Treatment
The goals in treating status epilepticus are to provide respiratory and cardiovascular support, terminate clinical and electrical seizure activity, identify and treat precipitating factors, and prevent systemic complications.137 Recognizing that a prolonged duration of seizure increases the risk of morbidity and mortality, the Epilepsy Foundation of America published a consensus view to initiate antiepileptic drugs for treatment 10 minutes after the onset of an episode of status epilepticus.138 A timetable for treatment of status epilepticus in children is provided in Table 42-2.
TABLE42-2 Suggested Timetable for Emergency Diagnosis and Treatment of Status Epilepticus
| Time | Exam/Intervention | Testing |
|---|---|---|
| Initial presentation: 0 min | Airway, breathing, circulation, IV access, monitoring | Glucose, oxygenation via pulse oximetry ± blood gas analysis |
| Primary survey: 5 min | Neurologic exam Administer antiepileptic drugs Lorazepam, 0.1 mg/kg IV Phenobarbital, 20 mg/kg IV Normal saline maintenance IV Reduce fever | Electrolytes, renal and liver function, ammonia, anticonvulsant levels, toxicology, complete blood cell count, urinalysis |
| Secondary survey: 15-30 min | Evaluate treatment results Second-line antiepileptic drug if seizure persists Fosphenytoin, 20 mg/kg IV; or phenytoin, 20 mg/kg IV | Patient-specific: cranial imaging (CT vs MRI), lumbar puncture, EEG, ECG |
| Status epilepticus: >30 min | Intubation and mechanical ventilation | |
| Refractory status epilepticus: >60 min | Titrate antiepileptic drug to burst suppression Pentobarbital, 10 mg/kg IV given over 30 min, then 5 mg/kg every hour for 3 doses, then 1 mg/kg/h; titrate to effect Midazolam, 0.15 mg/kg IV, then 1-2 µg/kg/min, titrate to effect Phenobarbital, 5-10 mg/kg IV every 20 minutes to achieve burst suppression, then every 12 hours Evaluate need for vasopressors | Continuous EEG Neurologic consultation Consider anesthesia consultation for treatment with inhaled anesthetic |
CT, computed tomography; ECG, electrocardiogram; EEG, electroencephalogram; IV, intravenous; MRI, magnetic resonance imaging.
Phenytoin/Fosphenytoin
In a study in adults comparing lorazepam, phenytoin, phenobarbital, and diazepam, phenytoin had the highest success rate in stopping status epilepticus.139 Phenytoin is not commonly associated with respiratory depression and has less of an effect on the impairment of consciousness than either benzodiazepines or barbiturates. Fosphenytoin has the advantage of having a faster infusion rate, shorter onset of action, and less cardiovascular side effects than phenytoin but is more expensive.
Lorazepam
In the same study in adults, lorazepam had the second highest success rate in stopping status epilepticus.139 Lorazepam can be administered rapidly, has a long duration of effect, and is effective even when administered rectally. Lorazepam produced less respiratory failure requiring intubation than diazepam in retrospective140 and prospective studies.141 Incidence of respiratory depression in these studies varied widely—between 3% and 76%. Support for selection of Lorazepam over diazepam was also shown in a recent Cochrane review.142
Additional Diagnostic Workup
Lumbar puncture is best performed early after presentation, but not in unstable patients or those who may have increased ICP. The decision to perform lumbar puncture should be guided by head CT. Otherwise, the type of neuroimaging used in infants and children with status epilepticus should be individualized, depending on history and physical findings. Both electrocardiography (ECG) and EEG are useful to investigate cause of status epilepticus (i.e., long QT syndrome or identifiable EEG patterns). EEG is also useful in titrating therapy (see later discussion).137
Drug Treatment for Refractory Status Epilepticus
Initiation of treatment for refractory status epilepticus should occur by 60 minutes, usually with neurologic consultation and with appropriate monitoring in a pediatric ICU or intermediate unit. These patients are mechanically ventilated, and seizures are typically treated with a variety of therapies, generally to induce burst suppression on continuous EEG. Most commonly, pentobarbital is used as a continuous infusion to treat refractory status epilepticus. Pentobarbital is given initially as a slow IV loading dose of 5 to 15 mg/kg, followed by an infusion rate of 1 mg/kg/h titrated to effect. There are differing opinions on when to begin to wean therapy, but it is generally recommended that about 12 hours of seizure cessation be attained before weaning the infusion.143 In children, placement of either a central venous pressure or pulmonary artery catheter is indicated to titrate fluid, inotropic, and/or pressor support. Pentobarbital use often requires the addition of inotropes or pressors. As an alternative to continuous barbiturate infusion, phenobarbital can be administered every 20 minutes (5–10 mg/kg IV) to achieve burst suppression, and then as a chronic therapy every 12 hours. A midazolam infusion has also been shown to be effective in refractory status epilepticus in some children (0.15 mg/kg IV bolus followed by infusion of 1–2 µg/kg/min). The infusion can be increased every 15 minutes if seizures are still present on continuous EEG or if burst suppression is not achieved. With this approach in one series, inotropic support was not required.144
Stroke
Epidemiology
Stroke in children is becoming increasingly recognized and now exceeds an incidence of 8 cases per 100,000 children per year.145 Substantial advances in our knowledge of this condition in children have resulted from the work of the Canadian Pediatric Ischemic Stroke Registry. Neonates account for about 25% of these cases. The increasing incidence is believed to result from improvements in diagnostic tools (MRI, computed tomography [CT], magnetic resonance angiography [MRA]) applied to the pediatric population and to increasing survival rates in infants and children with stroke risk factors (e.g., complex congenital heart disease, malignancies).
Etiology
As discussed, atherosclerosis is a key risk factor for stroke in adults. In pediatric and neonatal stroke, extracerebral risk factors contribute to about 75% of cases; however, the spectrum of risk factors differs from those seen in adults. DeVeber145 grouped the most common risk factors for childhood ischemic stroke into vascular, intravascular, and embolic categories (Box 42-2). The most common vascular risk factor has been reported to be transient cerebral arteriopathy.146 Post-varicella arteriopathy, migraine, traumatic carotid dissection, and vasculitis, such as moyamoya, are also important examples in this category. In the intravascular category, sickle cell anemia, sinus thrombosis, leukemias, and both acquired and congenital prothrombotic states are important examples. Dehydration and intravascular volume depletion increase stroke risk in these settings, which are of special importance in the pediatric ICU. There is an 84% incidence of an acute systemic illness and a 30% incidence of dehydration in cerebral sinovenous thrombosis in infants and children.147 Congenital and acquired heart disease in infants and children are the most important underlying causes of embolic stroke.145 The risk of stroke in children after surgery for congenital heart disease is about 1 in 250 cases.148