Chapter 10 Intravenous Therapy
Intravenous Fluids and Medications
Basic Principles of Fluid and Medication Administration
The human body is about 60% water by weight with about 40% found in the intracellular compartment and the remaining 20% found in the extracellular compartment. Metabolic rate determines the amount of fluid needed in healthy people; however, injury and disease can greatly alter the amount of fluid required. Elevated body temperature increases metabolic rate by 12% for each degree Celsius (7% for each degree Fahrenheit). Decreased intake from fasting or altered consciousness and increased losses from diarrhea and vomiting are other examples leading to fluid and electrolyte imbalances.1
• Water moves across semipermeable cellular membranes by the process of osmosis. Osmolarity (the osmolar concentration in 1 L of solution) and osmolality (the osmolar concentration in 1 kg of water) are often used interchangeably because 1 L of water weighs 1 kg.1
• Tonicity applies to the solutions being infused and how that solution will affect the size of the cells. Osmotic pressure of a solution causes water to move into or out of cells. Isotonic fluids have the same osmolality as intracellular fluids, between 280 and 295 mOsm/L. Thus isotonic fluids will simply increase the extracellular volume but will not produce any osmotic shifting of fluids into or out of the cell. Hypotonic fluids have an osmolality less than intracellular fluids and hypertonic fluids have an osmolality greater than intracellular fluids. Infusion of hypotonic fluids will cause fluids to move into the cells, resulting in swelling of the cell and possibly causing them to burst. Infusion of hypertonic fluids will cause fluids to move out of the cells, causing them to shrink.1 Changes in the cell size from osmotic shifting occur in the venous endothelium, resulting in inflammation and thrombosis at the point where the fluid enters the vein.2 This process drives the need for a central venous catheter when the required fluids are extremely hypotonic or hypertonic. One example is parenteral nutrition.
The Infusion Nursing Society Standards of Practice calls for restricting the osmolality of fluids and medications infused through peripheral veins to no more than 600 mOsm/L.5
• Another critical factor related to IV fluids and medication is the pH, the acidity or alkalinity of the solution. Most IV fluids have a pH of 5, a slightly acidic level that extends their shelf life; however, the pH of fluids ranges from 3.5 to 6.2.1 The pH of all solutions will affect the integrity of the venous endothelium and extremes will produce inflammation of the vein. An example of an extremely acidic drug is vancomycin and of an extremely alkaline drug is phenytoin.
The Infusion Nursing Society Standards of Practice states that the pH of fluids and medications infused through peripheral veins should be between 5 and 9.5
• Consider the vesicant or irritating properties of the solution and medications given intravenously. A vesicant medication will produce tissue damage if it leaks from the vein into the subcutaneous tissue; therefore, the nurse must determine absolute patency of the vein when administering these medications. This means observation of the site condition, palpation for tenderness, aspirating for a positive blood return, and listening to all patient complaints. This level of assessment is necessary with each dose of medication regardless of when the catheter was inserted. Examples of medications in this group are commonly thought of as oncology chemotherapy agents; however, vancomycin, nafcillin, promethazine, high concentrations of dextrose, all calcium preparations, sodium bicarbonate, and potassium solutions are also vesicants.
• Nurses must incorporate the step of checking compatibility and stability of fluids and medications as part of safe administration practices. Stability means the amount of time the drug will retain its original characteristics. Drug stability is affected by many factors such as pH, the number of additives in solution, the volume of dilution, time in solution, light, temperature, the sequence of drugs added to solution, and the fluid container. There are three different types of drug incompatibility or undesirable reactions when drugs or solutions come into contact. Physical incompatibility is seen when there are visible changes such as a precipitate formation, color change, or increased turbidity. Chemical incompatibility is a nonvisible change in the drug’s chemical structure. Therapeutic incompatibility occurs after infusion when two drugs have similar effects.4
Types of Parenteral Fluids
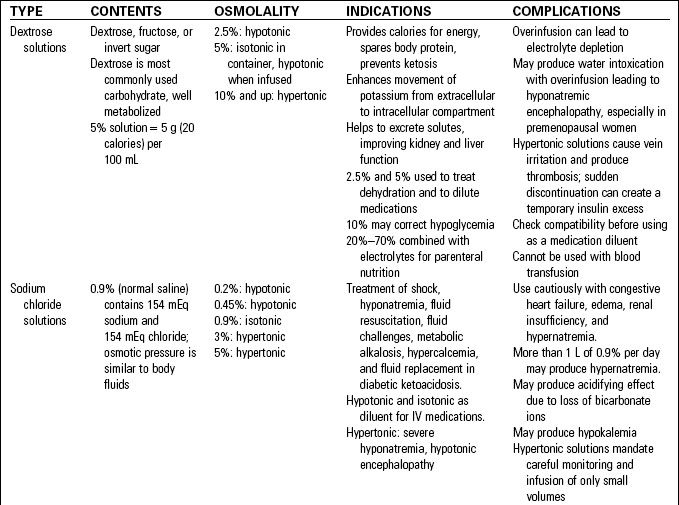
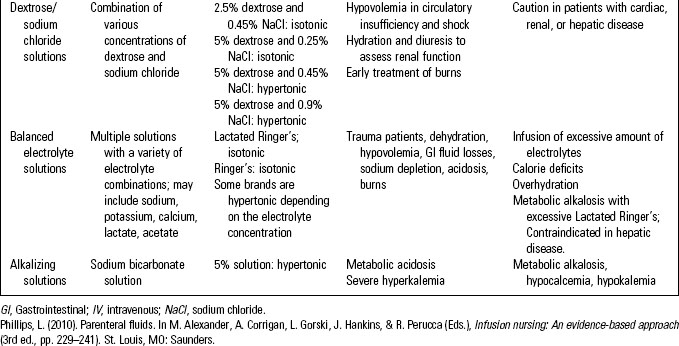
Intravenous fluids are classified as crystalloids or colloids. Crystalloid solutions contain solutes that mix and readily dissolve in solution. The dissolved electrolytes easily pass between intracellular and extracellular compartments. This group includes dextrose solutions, sodium chloride solutions, balanced electrolyte solutions, and alkalizing and acidifying solutions. (See Table 10-1.)
• Crystalloid solutions are available from a pharmacy compounding service or purchased as premixed solutions. After other medications (e.g., potassium chloride, heparin) have been added the solution is sterilized, providing a safer solution than those prepared by the nurse. While these premixed solutions save nursing time, the available concentrations should be limited to prevent medication errors.1 (See Table 10-2.)
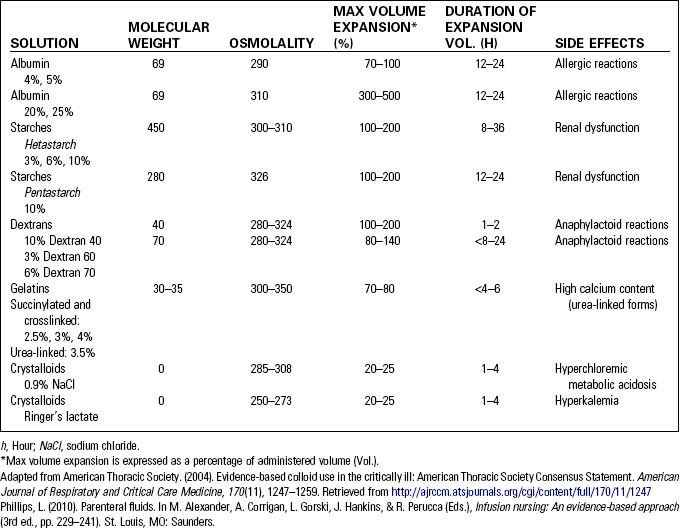
• Colloid solutions increase the intravascular volume and are also known as plasma expanders. These solutions pull fluid from the interstitial spaces. Albumin, dextran, hetastarch and pentastarch, and gelatins are included in this group. Although frequently used for fluid resuscitation in trauma, there remains controversy over the type and method of fluid to be used.1 Mannitol, also classified as a colloid, is an intravascular volume expander that acts as an osmotic diuretic.1 Table 10-1 compares common colloid and crystalloid solutions.
The Infusion System
Fluid Container
• Glass bottles were the original containers for IV fluids; however, plastic bags are now more common. Glass bottles require venting to allow air to enter and fluid to flow. Administration sets that contain a filtered air vent above the drip chamber are preferred. If vented administration sets are not available, the glass bottle can be vented by inserting a filter needle into the rubber stopper; however, regular needles should not be used for this purpose.5
• Plastic bags are collapsible and do not require venting to allow fluid to flow. Polyvinyl chloride (PVC), the original plastic in these containers, requires the addition of chemicals to make them soft and flexible. The chemical di(2-ethylhexyl)phthalate (DEHP) is now the cause of concern as this chemical can leach into the solution and be infused to the patient. Exposure to DEHP carries the greatest risk for male fetuses and infants, along with possible carcinogenic and hepatotoxic effects for others.5 Plastic fluid containers can have certain drugs adhere to the container’s surface. Up to 80% of the dose of insulin and nitroglycerin may adhere to the plastic and not be infused. Plastic containers made of polyolefin may eliminate this problem with many drugs; however, studies have shown that insulin will adhere to this plastic also. Use of glass bottles for infusing insulin may be required or, if plastic bags must be used, the nurse must carefully monitor the patient’s response to the infusion.5
• Syringes are also considered fluid containers and are used in combination with a syringe-loaded electronic pump. They are used for small volume drugs when the patient’s age (e.g., neonates) or condition (e.g., renal failure) cannot tolerate larger volume dilution of the medication.5
• Many drugs may be available in a use-activated container where the drug and diluent are in separate chambers. The divider between the chambers must be deliberately ruptured according to the manufacturer’s instructions. When the drug and diluent are not properly mixed together, only the diluent may infuse or a delayed rupture of the barrier may result in a rapid infusion of the undiluted drug.
• Some drugs require protection from light during their infusion. No solution containers are available to solve this problem; however, a simple paper bag placed over the fluid container will protect it from light. The disadvantage is that the fluid level is not easily visible.5
Administration Sets
• Primary continuous administration sets are used when the infusion is required for multiple hours, days, or weeks. It should be a single device to limit the number of connections. Once attached to the catheter hub, it should remain connected until it is time to be changed, usually no more frequently than 96-hour intervals but at least every 7 days according to the Centers for Disease Control and Prevention (CDC).
• Secondary sets are those sets of varying lengths that are used to deliver intermittent medications when continuous fluids are infusing. The secondary set should be attached to the primary continuous set and remain connected. Both the primary and the secondary sets are changed together, usually no more frequently than every 96 hours.
• Primary intermittent sets are those sets used to deliver intermittent medications when there is no primary fluid infusing continuously. These sets are connected and disconnected with each dose, thus increasing the risk of contamination. No studies have established a safe length of time for their use; therefore, the Infusion Nursing Standards of Practice states primary intermittent sets should be changed every 24 hours.3 The CDC Guidelines for the Prevention of Intravascular Catheter-Related Infections now state that the change interval for intermittent sets is an unresolved issue.6 The male luer end of the set must be maintained in a sterile manner when not connected to the catheter. This is accomplished by placing a new sterile cap securely on the end immediately after it is disconnected from the catheter. If there is any question about the integrity of this covering or the set, it should be discarded and a new set should be used.
• Certain infusions, such as nitroglycerin, fat emulsion, blood products, and arterial pressure monitoring, require special administration sets for safe infusion. The use of metered-chamber sets has decreased but these still could be useful in some situations. The chamber is located below the fluid container and will hold a small amount of fluid, usually 50 to 150 mL. When this volume has infused, the nurse must return to fill this chamber again. These sets have also been used to deliver intermittent medications; however, there is concern about ensuring that the chamber is properly labeled when the drug is infusing. Some infusion pumps will require a dedicated set while others may accept the general set used for all infusions. Know the specific type of set required for the infusion pump being used.
Flow Control
Gravity
Systems that depend on gravity include the traditional roller clamp and other manual flow regulators. The fluid container must be placed about 3 to 4 feet above the catheter site to create the pressure gradient and produce fluid flow. A standard roller clamp on a standard-bore administration set can be as accurate as plus or minus 10%, but numerous variables are involved. Changes in the distance between the container and the catheter, patient movement, and improperly stabilized catheters can reduce the accuracy to plus or minus 25%. Roller clamps should be placed on the upper third of the set length to allow for easy access and prevent patient manipulation. These clamps should be repositioned on the set periodically as they can produce a permanent kink in the set.5
Pressure cuffs are another example of manual flow control and are used for rapid infusion. The cuff is positioned around a plastic fluid container and inflated to exert pressure against the bag. Most systems have a warning on the dial when the maximum of 300 mm Hg or 6 psi has been reached.5
Electronic
Electronic infusion pumps come in many varieties, including pole-mounted volumetric pumps, ambulatory infusion pumps, syringe pumps, and patient-controlled analgesia pumps. The industry standard for this group is an accuracy rating of plus or minus 5%, but some may be as accurate as plus or minus 2%. Recent advances in electronic infusion pumps have included dosage error reduction systems and drug libraries. The nurse must understand the specific system in use and know how to operate it properly without bypassing these systems.5
With these infusion pumps it is critical to remember that some will continue to pump or force fluid flow regardless of the catheter or vein patency. The alarms are not designed to indicate when an infiltration or flow of fluid into the subcutaneous tissue has occurred.7 It is imperative that the catheter and infusion site be assessed frequently to avoid serious injury to the patient, especially prior to administering a medication. Many other challenges have been identified with the present design of electronic infusion pumps resulting in serious medication errors.8,9 It is imperative that nurses thoroughly understand how to operate electronic infusion pumps and that they do not rely totally on the machine to deliver accurate fluid flow constantly. The fluid container should frequently be assessed for fluid level and compared to the total volume infused to ensure that the patient is receiving the prescribed amount.
Other Components
• A short extension set added to a peripheral catheter will facilitate quick attachment of syringes and sets to the catheter without unnecessary pressure or manipulation of the catheter inside the vein. Extension sets may also be necessary to add length to the administration set.
• Filters may be integral to the administration set or be added on to the set. Filters are measured by the size of the opening or pores where the fluid passes. Filters with a 0.22-micron pore size are required on certain solutions such as parenteral nutrition, but other fluids such as fat emulsion are too large to pass through this small filter. When the parenteral nutrition solution is a total nutrient admixture (e.g., all components including the fat emulsion are mixed in one large container), a 1.2-micron filter must be used. Filters remove particulate matter, air, and some microorganisms.5
• Needleless connectors, previously called injection or access ports, are devices used to close the catheter hub while allowing for intermittent connection of administration sets and syringes. Needleless connectors are also found on the administration sets. There are multiple ways to categorize all of the devices in this group. Some are clear, allowing the nurse to observe for retained blood or drug precipitate, while others are colored and do not allow for this observation. Some have a straight fluid pathway while others have a complex internal mechanism and fluid pathway.
These devices may also be divided by how they are designed and how they function.10 Some are designed to use a blunt plastic cannula that is passed through a prepierced split septum. There are now two types of split septum systems that allow the direct attachment of a male luer of the syringe or administration set, thus eliminating the need for the blunt plastic cannula. Others are categorized as mechanical valves that are activated by the connection of the male luer of the set or syringe. The center post is pushed downward, opening the fluid pathway.
Fluid Displacement
• Negative fluid displacement devices will allow blood to reflux into the catheter lumen when the syringe or set is disconnected. If the blood is allowed to reside inside the lumen, it can become difficult to flush or become totally occluded.
• Positive fluid displacement devices will hold a small volume of fluid inside the device. Upon syringe disconnection, this small reserved fluid is pushed out to the catheter tip to overcome the blood reflux.
• Neutral fluid displacement devices do not allow blood to move into the catheter lumen on connection or disconnection.10
The technique for flushing and clamping these devices must be the correct one for the functionality of the device. Negative displacement devices require flushing the fluid into the catheter lumen, continuing to hold the syringe plunger, closing the clamp before disconnection, and then removing the syringe. This technique in that specific sequence will prevent blood from refluxing into the catheter lumen. This technique cannot be used with a positive displacement device. For those needleless connectors that have a positive displacement mechanism, flush the catheter, disconnect the syringe, and then close the clamp. For those devices with neutral displacement, the disconnection and clamping sequence can be done in either manner.10
There is growing concern about the risk of bloodstream infection associated with the use of these needleless devices. Some mechanical valves have been reported to produce an increase in catheter-related bloodstream infection. For this reason many guidelines now recommend the preference for a split septum device instead of some mechanical valves.6,11 Regardless of the type of needleless connector in use, all require thorough scrubbing with a disinfectant solution such as alcohol or chlorhexadine before each entry, and only sterile devices should be used to access the connector.6
Vascular Access Devices
Catheter Characteristics
Materials
Most catheters used currently are made of polytetrafluoroethylene (Teflon), polyurethane, or silicone. Teflon is relatively rigid, and this characteristic does not change after insertion. Polyurethane is a large family of materials known as thermoplastics. Once inside the body, polyurethane becomes softer because of body heat. While this family of material has superb physical strength, some formulations are limited by their chemical compatibility. Some older formulations do not tolerate contact with alcohol, while newer formulations do not have this same restriction. Silicone is a material widely used in catheters. It is extremely flexible and soft, but these characteristics require special methods for insertion. All catheter materials are tested for cytotoxicity, allergic reactions, their potential to produce inflammation, and hemocompatibility.5
Diameter
Catheters are measured in several ways. The outer diameter is a measurement from the outside wall through the center of the lumen to the outside wall on the opposite side. This measurement is critical to compare to the internal diameter of the vein lumen. The catheter should not consume more than about a third of the vein lumen. The outer diameter measure is taken in millimeters. Multiplying this measurement times three yields the French size. Thus a 12 French catheter will have an outer diameter of 4 mm.5
The internal diameter is measured from the inner wall through the center of the lumen to the inner wall on the opposite side. This measurement produces the internal volume of the catheter, the maximum flow rate through the lumen, and the amount of pressure the catheter can tolerate. The measurement of internal volume applies to the size as it comes from the manufacturer. If the catheter length has been trimmed, the internal volume has changed.5
Lumens
Many central venous catheters have multiple lumens. These are separate channels throughout the length of the catheter and are used to infuse multiple solutions simultaneously. Some catheters have these lumens exiting into the bloodstream at the same point, while others have staggered lumen exit points. Hemodialysis catheters have the lumen exit points staggered by at least 2 cm, but infusion catheters with staggered lumens may have less than 1 cm of separation. The tip of all central venous catheters is located in the superior vena cava where the blood flow is approximately 2000 mL per minute. This rapid flow rate will usually provide sufficient hemodilution to prevent mixing of incompatible medications at the catheter tip.5