Fig. 21.1
Tracing of SSEPs from left upper extremity, with normal responses from Erb’s point and the cervical region, but abnormal from the cortical leads. These abnormal tracing were caused by faulty positional placement of the C4 cortical lead. Normal responses were obtained after proper positioning
As surgery commenced, all neurophysiologic parameters were within normal limits. The dura was opened and the microscopic portion of surgery started. Surgery progressed well and the surgeon performed a few surgical adjustments to the surgical field to facilitate good exposure of the aneurysm. The technologist then announces an isolated but significant drop in the amplitude of the TCMEP signals recorded from the left hand (Fig. 21.2).
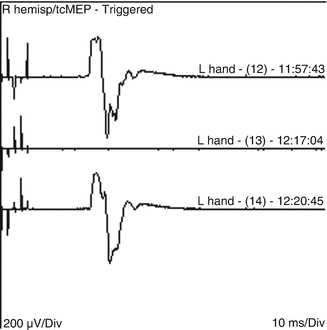

Fig. 21.2
Loss of hand TCMEP due to retractor pressure with prompt recovery after releasing the retractor
What is the Cause of This Change?
With any change in a signal during a surgical procedure, a surgical cause must be sought out quickly and efficiently—surgical causes must be quickly reversed before permanent damage occurs. Since surgical maneuvers were being performed with multiple adjustments of the retractors, this was likely the culprit. Retractor pressure can alter blood flow from the lenticulostriate arteries that supply the deep cortical and subcortical motor and sensory pathways or the branches of the anterior or middle cerebral arteries that feed the motor and sensory strips [43].
As noted above, TCMEP responses are more sensitive to ischemia and precede SSEP changes [21, 29, 44]. While the presence of SSEPs may be assuring of the outcome, the TCMEP changes serve as an early warning system for possible damaging ischemia [45]. In this case, the release of the retractor resulted in fast recovery of the TCMEP signals. To make sure no other source of change in TCMEP is overlooked, it is important, as always, to scan the rest of the possible causes of a unilateral change in a TCMEP. An isolated change is unlikely to be related to anesthesia or physiological maneuvers; since position was not altered, if there is no new pressure on the left upper extremity, this cause can be excluded too. Simple evaluation of the stimulation pattern and the impedance of the stimulating electrodes, which were unchanged from baseline, can also rule out technical causes.
The surgical exposure proceeded well, and the surgeon requested initiation of pharmacologic burst suppression to, in theory, prolong the tolerance for ischemia in preparation for possible temporary clip placement [18, 46–48]. (Of note, there is no solid clinical evidence that pharmacologic treatment of ischemic brain and/or agents used intraoperatively for burst suppression is effective in these patients.) [11, 18]. Burst suppression was achieved by increasing the propofol infusion to 150 μg/kg/min. New “baseline” neuromonitoring signals should be obtained before critical surgical maneuvers, since high doses of most intravenous anesthetics can affect evoked potentials [49]. The surgeon took a final look and placed two tandem permanent clips. Two minutes later, the TCMEP signals of the left hand deteriorate followed by left median nerve SSEP changes.
What is the Cause of These Changes?
Since these unilateral changes occurred after surgical manipulation, surgical causes for such change are highly likely and should be investigated and managed immediately [21, 29, 45]. All members of the team should be involved in the investigation. The technologist should check the machine to confirm the fidelity of the system and exclude any last minute technical error. The anesthesiologist should induce hypertension (we usually raise blood pressure by approximately 20 %) to increase perfusion via the collateral circulation (i.e., the leptomeningeal pathways). The surgeon will visually inspect the field for potential vascular compromise, use a Doppler to interrogate blood flow, and may use indocyanine green (ICG) to perform a noninvasive angiogram (see Chap. 22). In this case, the ICG angiogram showed clip impingement on one of the blood vessels [50, 51]. The clip was readjusted with immediate recovery of TCMEPs followed by recovery of SSEPs (Fig. 21.3) (Video 21.2).
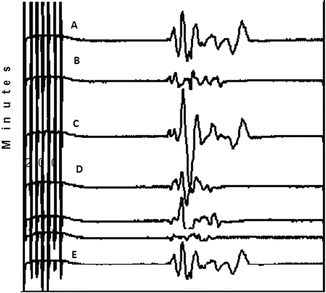

Fig. 21.3
Basilar artery aneurysm ; A = baseline, B = clip positioned, C = clip removed, D = clip repositioned, E = clip repositioned again
When a permanent clip is placed, there is risk of occluding a perforating artery, decreasing the size of the parent artery or causing severe spasm. Changes in TCMEP/SSEP can help identify such changes related to ischemia and a quick readjustment and treatment may prevent postoperative neurologic deficits [21, 29]. After clip readjustment and recovery of the signals, ICG and the Doppler confirmed good blood flow. The surgery concluded uneventfully. The patient emerged smoothly and was extubated in the operating room with the patient still receiving an infusion of remifentanil 0.05 μg/kg/min. Immediate postoperative exam revealed no neurological deficits. Fentanyl was administered as needed for postoperative analgesia and the patient was taken to the recovery room before proceeding to the ICU for overnight monitoring.
Case Presentation 2: ICA/Ophthalmic Aneurysm
An otherwise healthy 57-year-old female is scheduled for craniotomy for clip ligation of an unruptured 12 mm right internal carotid artery aneurysm just distal to the ophthalmic artery.
Anesthesia proceeded as in the first case, above. The surgical plan included potential placement of a right internal carotid artery endovascular balloon for occlusion with suction decompression to facilitate aneurysm clipping [52]. Both groins and the right neck were prepped and draped prior to starting the craniotomy.
Should Only Upper Extremity SSEPs and TCMEPs, Only Lower Extremity SSEPs and TCMEPs, or Both Upper and Lower Extremity SSEPs and TCMEPs be Monitored for This Surgery?
With intracranial surgery, it is important to monitor the territory of the vascular structures where ischemia is most likely to occur [53]. For aneurysms involving the internal carotid artery (such as this case), middle cerebral artery, or posterior circulation, ischemia is most likely to involve the vascular territory of the sensory cortex of the contralateral upper extremity [44]. Hence, upper extremity SSEPs and TCMEPs are the most appropriate monitoring modalities. If the aneurysm involved the anterior cerebral artery or the anterior communicating artery, the contralateral lower extremity would be at primary risk [44]. With this surgery, the anterior circulation may also be at risk, and thus, monitoring the lower extremities should also be performed. In reality, since multiple vascular territories can be affected during intracranial neurovascular procedures in general, the use of both SSEP and TCMEP monitoring for the upper and lower extremities should be routinely considered [54].
Baseline-evoked potentials for upper and lower extremity SSEPs and TCMEPs were robust in amplitude and normal in latency and morphology. Surgery proceeded uneventfully with exposure of the aneurysm. The decision was made to proceed and place an endovascular balloon into the intracranial internal carotid artery proximal to the aneurysm via the right femoral artery. This was done with a plan to inflate the balloon, suction deflate the aneurysm, and trap the aneurysm with a temporary clip placed distal to the aneurysm on the intracranial internal carotid artery.
Trapping an aneurysm refers to the temporary occlusion of both antegrade blood flow from the upstream, parent artery, and retrograde blood flow from the distal, collateral arteries, in order to allow tension in the aneurysm neck to decrease enough for clip ligation and possible parent artery clip reconstruction. For anterior communicating artery aneurysms and middle cerebral artery aneurysms, trapping involves placement of three to four temporary clips. In contrast, for intracranial proximal paraclinoid carotid artery aneurysms, either a temporary cross clamp has to be placed on the extracranial internal carotid artery after exposure by neck dissection, or an endovascular balloon tip catheter has to be placed in the internal carotid artery, proximal to the aneurysm, and the balloon inflated to occlude antegrade blood flow (Fig. 21.4). A more distal intracranial internal artery temporary clip is still required to occlude retrograde blood flow into the aneurysm. Suction can be applied in this scenario via the endovascular catheter to decompress the aneurysm while retrograde blood flow continues [52]. These maneuvers will enable the surgeon to safely clip the aneurysm, but with the risk of ischemia to any territory that is perfused by perforator arteries within the trapped arterial segment (e.g., the recurrent artery of Huebner during trapping for an anterior communicating artery aneurysm or the opthalmic artery during trapping of a paraclinoid carotid artery aneurysm). There is also the potential for ischemia to the territory normally supplied by the trapped parent artery, unless collateral flow via the Circle of Willis and/or the leptomeningeal pathway arteries is adequate.
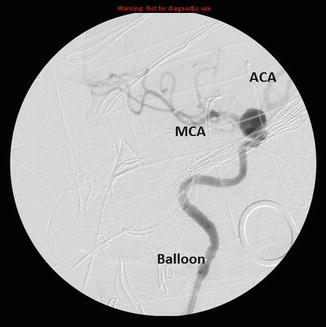

Fig. 21.4
Angiogram showing the placement of the balloon in the ICA, the aneurysm and both the MCA and ACA
Although traditionally both burst suppression and hypothermia would have been applied for this case, there is a dearth of literature support for these activities. In theory, decreasing the oxygen consumption of the brain, along with increasing collateral blood flow, may prolong the time that the brain can tolerate ischemia [18, 47, 48]. Administering an intravenous hypnotic or a volatile anesthetic decreases oxygen consumption of neuronal tissue associated with electrical activity. However, once the EEG achieves a burst suppression ratio of 0.8 (i.e., an EEG pattern of 80 % electrical silence and 20 % bursts of activity), there is little additional decrease in the cerebral metabolic rate and the cerebral blood flow [55]. Hypothermia can also decrease cerebral oxygen consumption, with the added benefit of decreasing both the cellular metabolism associated with electrical activity and the energy requirements for nonelectrical cell processes [56]. Therefore, hypothermia may produce additional tolerance to cerebral ischemia as the temperature is lowered below the point of electrical silence [57]. Unfortunately, mild intraoperative hypothermia (33.5 °C) did not change the neurologic or cognitive outcomes in patients who had World Federation of Neurologic Surgeons Grade I or II SAH when compared with normothermia in a large international, multicenter, double-blinded, randomized-controlled study (Intraoperative Hypothermia for Aneurysm Surgery Trial [IHAST]) [58]. In addition to prolonging the tolerance to ischemia during aneurysm trapping, hemodynamic maneuvers that increase the collateral blood flow (e.g., increase systemic mean arterial pressure, decrease brain bulk, increase brain luxury perfusion) may prevent the onset of ischemia [18]. In this case, we achieved burst suppression and increased the blood pressure.
Surgery proceeded with the balloon inflated. The EEG demonstrated a burst suppression ratio of 0.8, and both the TCMEP and SSEP signals were stable. The aneurysm was difficult to clip and it took extra time to be completed. Twelve minutes after initiating the trapping, TCMEP signals from both the left arm and the left foot deteriorated, followed by SSEP signal deterioration at 15 min. Signal deterioration was thought to be related to cerebral ischemia from trapping. The surgeons were notified; unfortunately, they had opened the aneurysm dome and therefore reperfusion at that time was not an option (Video 21.3). The mean arterial pressure was increased approximately 20 %. Surgery proceeded with complete loss of both TCMEP and SSEP signals after 2 more minutes despite further induced hypertension. With completion of the aneurysm clip ligation and clip reconstruction of the parent artery, the balloon was deflated and the distal temporary clip was removed. The TCMEP and SSEP signals gradually recovered to baseline morphology. Visual inspection, Doppler testing, and ICG angiograms demonstrated satisfactory reconstruction of the parent artery and no areas of inadequate dye or Doppler flow. Surgery proceeded and the patient was awakened at the end of surgery. On wake-up, it was noted that the patient had mild weakness on the left side, more in the arm than the leg, and both improved within 30 min.
In this case, the TCMEP and SSEP changes occurred at minutes 12 and 15, respectively. These late occurring post-trapping changes are likely better tolerated than changes that may occur within the first 4 min after temporary arterial occlusion or aneurysm trapping [21, 29, 44]. The changes seen in both TCMEP and SSEP are due in this case to surgical maneuvers consistent with the aneurysm trapping. In general, all other causes should be contemplated and excluded. However, because of the temporal relationship to the known surgical insult, we needed to concentrate on the surgical cause. It is important to have a stable anesthetic and hemodynamic course and to avoid changes in technical parameters at this point of surgery in order to avoid producing false-positive signal deterioration.
The initial weakness in the first 30 min after emergence from anesthesia may be related to regional ischemia caused by blood flow interruption and alterations in regional cerebral blood flow, especially in areas of ischemia. Reduced reperfusion may result in slower washout of the anesthetic drug, thereby prolonging the time course until the region at risk recovers from anesthesia [59].
Case Presentation 3: Basilar Apex Aneurysm
A 64-year-old woman is undergoing an orbitozygomatic-pterional craniotomy for microsurgical clipping of an unruptured 15-mm wide-neck basilar apex aneurysm.
How Does This Aneurysm Location Influence the Choice of Neurophysiologic Monitoring Modalities to be Used?
Part of the large morbidity and mortality associated with surgical correction of basilar apex and posterior circulation aneurysm is due to the proximity of multiple brainstem and subcortical perforator arteries as well as the limited surgical corridors, which make visualization challenging [17]. Although SSEPs and TCMEPs provide information regarding the integrity of the subcortical and brainstem pathways with regard to direct neurologic injury or injury secondary to ischemia, brainstem auditory-evoked responses (ABRs) provide additional monitoring more specific to the brainstem integrity, and ABRs are very resistant to anesthetic effects (see Chap. 3 [“Auditory Evoked Potentials”] for additional information). Therefore, ABRs are often included to give complimentary information to help determine the specific location of neurologic trespass that may produce permanent neurologic injury from trauma or ischemia. In addition, because it is difficult to obtain a panoramic view of basilar apex aneurysms and the perforators at risk for injury, using these neuromonitoring modalities provides better functional evaluation of the possibility of clip impingement of perforators (Fig. 21.5). Electromyography (EMG) for cranial nerve monitoring of any cranial nerve with a motor component at anatomic risk may also be used for posterior circulation aneurysms (see Chap. 7, “Electromyography”, for additional information.)
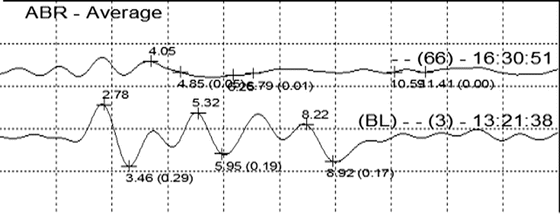

Fig. 21.5
ABR tracing showing the changes in waves IV and V
After isolating the aneurysm, the surgeon determined that the aneurysm neck was extremely tense and that there was no safe location to place a temporary arterial clip to soften the aneurysm neck. After the aneurysm had been exposed and the patient demonstrated an EEG with a burst suppression ratio of >0.8, as requested by the surgeon, the surgeon manipulated the aneurysm to inspect possible clip placement and inadvertently ruptured the aneurysm. The microsurgical field filled with blood and it was impossible for the surgeon to identify any anatomical structures to control the bleeding. The anesthesiologist administered 0.4 mg/kg of adenosine as a rapid intravenous bolus and this produced a 15 s sinus pause and a total of 45 s of profound hypotension (systolic blood pressure <60 mmHg), allowing a bloodless field and permanent clip placement [15] (Video 21.4 and Video 21.5).
All neurophysiologic signals were stable. The surgeon evaluated the position of the clip and was satisfied. As the surgeon irrigated the field to prepare to begin dural closure, the left upper extremity TCMEPs became attenuated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








