17 Daniel Hutton, Javed Siddiqi, and Dan Miulli Elevated intracranial pressure (ICP) remains a frequently encountered dilemma in the neurosurgical intensive care unit (NICU). Few other pathologies challenge clinicians’ insight and vigilance as does intracranial hypertension. In addition, treatment is limited because of a lack of true understanding and randomized trials for documentation of outcome. Intracranial hypertension is seen in 50% of those with intracranial mass lesions and 33% of those with diffuse injuries.1 ICP is a function of the contents of the cranial vault. The sum of the volumes of blood, brain, cerebrospinal fluid (CSF), and other elements (tumor, hematoma, abscess, edema) in an inelastic bony cranial vault together comprise the ICP. This is known as the Monro-Kellie hypothesis.2 Therefore, an increase in any of the intracranial elements causes a concomitant decrease in the other elements. This principle does not apply to children with unfused sutures or to patients with comminuted skull fractures. The intracranial fluid model in a subject without head trauma has shown that adequate perfusion, or cerebral blood flow (CBF), is exquisitely autoregulated and relatively constant, with small amounts of flux. Numerous variables are available to monitor and manipulate in the NICU. Tenets are based on knowledge of the cardiopulmonary system, specifically, cardiac output. In this, adjustments of vasopressors, inotropes, chronotropes, and other medication are made to optimize cerebral perfusion. Though usually requiring invasive monitoring, cardiac output may be approximated with a simple calculation: where SV is stroke volume, HR is heart rate, VO2 is oxygen consumption, and AVDO2 is arteriovenous oxygen content difference. CBF is measurable with neuroimaging modalities, including xenon CT, positron emission tomography (PET) scanning, and functional magnetic resonance imaging (MRI). These techniques are quite expensive and usually unavailable on a continuous basis to most clinicians. CBF is dependent on cerebral perfusion pressure (CPP), which is the net driving hemodynamic force with consideration of ICP. Normal adult CPP is >50 mm Hg. CBF is related to CPP via Poiseuille’s law. Using this formula, CPP is directly proportional to CBF and also to vessel radius; it is inversely proportional to blood viscosity and vessel length. Shown mathematically, Poiseulle’s law is where r is vessel radius, n is viscosity, and l is vessel length. CPP = MAP – ICP, where MAP is mean arterial pressure. where DBP is diastolic blood pressure and SBP is systolic blood pressure.
Intracranial Pressure Fundamentals
 What Is Intracranial Pressure?
What Is Intracranial Pressure?
 Cerebral Blood Flow and Intracranial Pressure
Cerebral Blood Flow and Intracranial Pressure
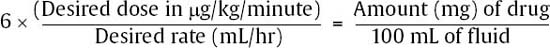
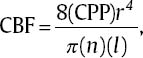
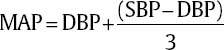
| cm CSF x 1.36 = mm Hg |
| Dependent upon atmospheric pressure (varies with altitude), hydrostatic pressure, and filling pressure |
| CSF pressure needs to be 3 to 5 mm Hg higher than venous pressure for absorption. |
| Adults and older children 5 to 15 mm Hg 6.5 to 19.5 cm CSF |
| Young children <3 to 7.4 mm Hg |
| Term infants <1.5 to 5.9 mm Hg |
| CSF pressure decreases 0.5 to 1.0 cm CSF for every milliliter of CSF removed. A minor decrease in pressure suggests hydrocephalus, whereas a large drop in pressure may signify tumor. |
CSF, cerebrospinal fluid.
Optimization of CPP at 60 to 70 mm Hg has been more reliably shown to be associated with an improved neurologic outcome. Normal ICP is age dependent (Table 17–1).
Although many definitions for a pathological threshold of ICP have been given, 20 to 25 mm Hg is generally accepted as truly pathological.3
 Etiology of and Findings Associated with Intracranial Hypertension
Etiology of and Findings Associated with Intracranial Hypertension
The causes of intracranial hypertension include the following:
- Cerebral edema
- Hyperemia (loss of autoregulation)
- Hematoma: epidural hematoma (EDH), subdural hematoma (SDH), intracerebral hemorrhage (ICH), foreign body, or combination with depressed skull fracture
- Hydrocephalus
- Communicating from post-traumatic, secondary to aneurysmal subarachnoid hemorrhage (SAH) or arteriovenous malformation (AVM), meningitis
- Obstruction from tumor and aqueductal stenosis
- Communicating from post-traumatic, secondary to aneurysmal subarachnoid hemorrhage (SAH) or arteriovenous malformation (AVM), meningitis
- Hypercarbia (minute ventilation is too low or impaired alveolar gas exchange)
- Low minute ventilation
- Impaired gas exchange: hemothorax, pneumothorax, and pulmonary contusions
- Pneumonia
- Acute respiratory distress syndrome (ARDS)
- Low minute ventilation
- Venous obstruction/thrombosis
- Agitation (increased intrathoracic and intra-abdominal pressures)
- Status epilepticus (may be without overt tonic-clonic activity)
- Vasospasm
- Hyponatremia
The following findings are associated with intracranial hypertension:
- Drowsiness → Somnolence → Obtundation
- Nausea/vomiting
- Blurred vision or diplopia
- Cushing’s triad:
- Hypertension
- Bradycardia
- Respiratory irregularity
- Hypertension
- Motor or sensory deficits
- Cranial nerve (CN) palsies: CN III for uncal herniation; CN VI for acute hydrocephalus; CN VI and VII for enlarging cerebellar hemorrhage4
 Monitoring Intracranial Pressure
Monitoring Intracranial Pressure
Previously, much confusion existed regarding which patient populations would benefit from monitoring ICP. Indications for ICP monitoring were refined in 1995 by Bullock et al.3 They include GCS ≤ 8 (postresuscitation) and abnormal noncontrast brain CT5 or normal brain CT, but with at least two of the following: age > 40 years, SBP < 90, decerebrate or decorticate posturing on motor exam.
Relative contraindications to ICP monitoring include coagulopathy (International Normalized Ratio [INR] > 1.3), anoxic injury (“postcode”), and metabolic causes of coma, including intoxication (Table 17–2).
Types of Monitors
Various forms of monitoring have been used to assess ICP. Currently, intraventricular catheters are by far the most common. Their usefulness is twofold: proper assessment of ICP with less drift than other modalities, and the ability to treat intracranial hypertension by evacuation of CSF. Other methods include intraparenchymal, subarachnoid, subdural, and epidural bolts and, in infants, fontanometry.
| Indications | Contraindications |
|
|
GCS, Glasgow Coma Scale; ICP, intracranial pressure.
Intraventricular catheters are typically placed at Kocher’s point in the frontal lobe. Other sites commonly used for ventriculoperitoneal shunts may be used, including Keen’s point, Dandy’s point, and Frazier’s point. Landmarks exist for frontal lobe placement of intraventricular catheters. Generally, placement is performed on the nondominant side, 1 to 2 cm anterior to the coronal suture.
Procedure
Kocher’s point is located 12.5 cm posterior to the nasion in the sagittal plane, then 2.5 cm lateral to midline. A small patch of hair is shaved, and the sterile site is prepped. After infusing lidocaine with epinephrine into the subcutaneous tissue and periosteum, a 1 to 2 cm linear incision is made. The hand drill is introduced in the trajectory of the opposite medial canthus of the eye and approximately 1 cm anterior to the tragus. After bone purchase is made through both tables of the skull, the drill is removed, and the dura is incised in a cruciate manner. A catheter is placed in the same trajectory as the drill, with the catheter advanced approximately 4 to 6 cm. A palpable “pop” through the ependymal surface is often felt, and retrieval of CSF is seen. Depending on the system used, the system is secured into place and attached for monitoring and drainage of CSF. Sterile dressings are then applied.
Intraparenchymal pressure monitors may also be placed with the above described procedure and placement of the parenchymal fiber 2 to 3 cm into the brain substance.
At many locations, triple lumen ventricular catheters are used and are supplemented with microdialysis catheters, temperature, pH, and oxygenation probes. This has provided advanced therapeutic models for optimization of multiple variables. With the exception of brain tissue oxygen sensors, improved outcome has yet to be determined.
Waveforms
Normal waveforms are rarely seen due to changes associated with the traumatic population. However, the tallest peak of the ICP wave corresponds to the atrial systolic wave. The smaller peak corresponds to the A wave on the central venous pressure (CVP) waveform.
Pathological waves are due to alterations of CPP, whether resulting from increased ICP or decreased MAP, or both. An increase in ICP is thought to be associated with a sharpened appearance of the waveforms. An increase in venous pressure, conversely, has a more rounded appearance (Table 17–3).
In 1960, Lundberg described several pathologies and associated changes in waveform characteristics.6 Lundberg A waves (plateau waves) are usually seen with ICP >50 mm Hg. It has been postulated that there may be an associated increase in MAP. Lundberg B waves (pressure pulses) are described as an amplitude of 10 to 20 mm Hg and are associated with various types of breathing. Lundberg C waves have a frequency of 4 to 8/minute and have been seen in normal pressures as well as with Lundberg A waves in the premorbid state (Table 17–4).
| Normal ICP waveform | ||
| Peak | Wave | Origin |
| First large peak | Percussion wave W1 (pulsatile) | Systolic pressure, large intracranial arteries and choroid plexus CBF |
| Second small peak | Tidal wave W2 (pulsatile) | Central venous wave from right atrium, from brain increased elastance/decreased compliance |
| Inverted | Inverted | Miscalibrated monitor |
| Third small peak | Dicrotic wave W3 | Arterial pulse |
| Expiration | Increases overall wave | Increasing central venous pressure |
| Inspiration | Decreases overall wave | Decreasing central venous pressure |
| Increased ICP waveform | ||
| Peak | Change with increased ICP | |
| First large peak | Increases slightly | |
| Second small peak | Increases disproportionately to first wave | |
| Third small peak | Increases disproportionately to first wave | |
ICP, intracranial pressure; CBF, cerebral blood flow.
Lundberg A wave (plateau wave) Mean wave > 50 mm Hg Entire wave lasts 5 to 20 minutes, then returns to slightly elevated baseline. Increased cerebral blood volume from low CPP, then vasodilation, increasing ICP, lowering CPP, causing ischemia, and resulting in brainstem response. Occurs when ICP exceeds the limits of cerebral compliance; reflects ischemia |
Lundberg B wave (pressure wave) Mean wave > 20 to 50 mm Hg Entire wave lasts over ½ to 3 minutes. Possibly not due to increased ICP; may be due to respiratory changes and variations in CBF Can be seen in sleep Suggests that Lundberg A (plateau) waves may form |
Lundberg C wave (preterminal wave) Mean wave < 20 mm Hg Entire wave increased every 10 seconds ICP transmission of cyclic variation in SBP |
CBF, cerebral blood flow; CPP, cerebral perfusion pressure; ICP, intracranial pressure; SBP, systemic blood pressure.
 Surgery to Relieve Intracranial Hypertension
Surgery to Relieve Intracranial Hypertension
Decompression of mass lesions is of critical value. Unfortunately, only guidelines exist to help govern surgical intervention. Surgery previously was performed on an exploratory basis. Exploratory bur holes were made ipsilateral to the pupillary abnormality or contralateral to the worst motor response. If blood was encountered, a full craniotomy was performed. Advances in imaging techniques have antiquated this technique.
Principle evacuation of the mass lesion is paramount. However, one should remain keen to the possibility of craniectomy. A large incision and flap should be turned for maximal exposure for visualization and the possibility of allowing for cerebral edema should bone flap be left off. The general size of an adult bone flap should be at least 11 × 15 cm.
< div class='tao-gold-member'>







