12 Dan Miulli Pain must be treated. Neglecting pain treatment results in undue suffering, anxiety expressed by the patient and the family, fear, anger, depression, slow recovery, decreased ability to participate in activities of daily living, weight loss, fever, increased heart and respiratory rates, increased blood pressure, chest pain, myocardial infarction (MI), atelectasis, constipation, and infection.1–3 Overmedication results in the inability to perform a neurologic exam, which is the most important means of following a patient with neurologic disease or injuries. Overtreatment of pain and sedation leads to prolonged intubation, prolonged intensive care unit (ICU) stay, pneumonia, and deep venous thrombosis (Table 12–1).4 In a severe head injury or comatose patient who is intubated, only subtle changes may foretell drastic intracranial changes and problems such as contusions blossoming or other small abnormalities leading to midline shift and herniation. If the patient is overmedicated, a treatable lesion will be missed. Frequently, in patients with severe head injury or severe neurologic deficits, an intracranial pressure (ICP) monitor is inserted; however, the ICP, although rising and falling with movement, suctioning, and pain, will change exponentially as the brain has reached the ability to compensate. A gradual change in neurologic exam precedes large changes in ICP. Therefore, there must be a fine line that is maintained between pain and sedation, and under- and overmedication in the neurologic patient.
Sedation and Pain Management in
the Neurosurgical Intensive Care
Unit Patient
 General Principles of Pain Management
General Principles of Pain Management
| Undertreatment of pain | Overtreatment of pain |
| Suffering, anxiety, fear, anger, depression | Inability to perform neurologic exam |
| Slow recovery | Prolonged intubation |
| Decreased ability to participate in ADL | Prolonged ICU stay |
| Patient’s family anxiety | Pneumonia |
| Weight loss, fever, increased heart and respiratory rates, increased blood pressure, chest pain, MI; atelectasis, constipation, infection | DVT |
ADL, activities of daily living; DVT, deep venous thrombosis; ICU, intensive care unit; MI, myocardial infarction.
 What Is Pain?
What Is Pain?
Pain is “whatever the experiencing person says it is, existing whenever s/he says it does.”5 It is an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.6 In biological terms, stimulus energy from any source is converted into nerve impulses and transferred from the site of transduction to the central nervous system (CNS) and brain, during which time the signal is modulated at multiple levels before being perceived at the highest centers of the brain.
Nociceptors are specific receptors that respond to tissue trauma or stimuli that may cause tissue trauma.7 They are located in skin, connective tissue, muscle, tendon, muscle spindle, joint capsules, bone, and viscera, and around nerves and blood vessels. Thankfully, they are not located in the brain substance itself. The tissue changes from injury-released prostaglandins, substance P, bradykinins, cytokines, histamine, and serotonin. This is the first area where pain can be modulated. The impulses from the nociceptors responding to these substances or responding to simple mechanical deformation travel along slow conducting unmyelinated C fibers or faster conducting, small myelinated A delta fibers. Although both C fibers and large A delta fibers respond to pain, it is the ratio of response that determines the passage through the dorsal horns as pain; the response from C fibers must be greater than the response from A delta fibers. The impulses are mostly from the trunk and limbs, and therefore go to the dorsal horn of the spinal cord, usually activating N-methyl-D-aspartate (NMDA) receptors, the second site of pain modulation. NMDA receptor stimulation causes intraneuronal elevation of Ca2+, which stimulates nitric oxide synthase (NOS) and the production of nitric oxide (NO).8 Substances known to block NMDA receptors include d-methadone, dextromethorphan, ketamine, naloxone, and amantadine. It is at the NMDA receptors that inherent spinal cord nociceptor modulators such as γ-aminobutyric acid (GABA), serotonin, glutamate, substance P, norepinephrine, and endogenous opioids are released by spinal interneurons in laminae I, II, IV, and V of the dorsal horn, which are controlled by brain cortex centers, periaqueductal gray, or concurrent spinal cord input. The modified signal crosses in the spinal cord and ascends in multiple tracts, such as the spinoreticular, spinothalamic, spinomesencephalic, and spinohypothalamic, to the reticular formation, thalamus, mesencephalon, and hypothalamus, respectively, the third area of modulation. Sensations reaching the thalamus undergo complex modulation to determine if the sensation will be processed to reach the brain. From these intermediates, the signal passes to the frontal cortex, insular cortex, limbic structures, and sensory cortex to label the pain as good or bad, the fourth area of possible modulation.
A functioning brain is needed for the perception of pain. Each area through neurochemical release, such as GABA, norepinephrine, substance P, serotonin, and opioids, influences the pain. These same areas respond to memories, social influences, environmental influences, experiences, depression, and culture. Repeated stimuli at any location further change the characteristics of the pain and the threshold (Table 12-2).
Acute injury is mostly transmitted as nociceptive pain. This differs in treatment from neuropathic pain associated with chronic repeated stimuli and caused by aberrant signal processing in the peripheral or central nervous system. After repeated stimulation, the pathophysiological abnormalities of neuropathic pain become unrelated to the provocative event. This type of pain occurs infrequently in the ICU but may be seen in patients with multiple strokes, multiple fractures, or cancer pain.
 Assessment of Pain
Assessment of Pain
Pain management depends on an appropriate assessment of the pain most often recorded on a pain scale. Outpatient and pain assessment in the awake cooperative patient requires obtaining a detailed history along with the examination. The history of the awake cooperative patient must include past medical and surgical history, medications, smoking and alcohol intake, family history, psychosocial history and review of symptoms, and physical examination. These areas will help identify physical and psychological modifiers of the pain, which is important when considering nonnarcotic and nonmedicinal treatments, such as ice, elevation, antidepressants, and environmental factors. The medical and surgical history is important when evaluating the patient for contraindications to types of therapy.
| Area in pain pathway | Neurochemical | Possible modulation |
| Nociceptor | Prostaglandins, substance P, bradykinins, cytokines, histamine, and serotonin | Local anesthetics, ASA, NSAIDs, opioids, acetaminophen, AEDs, and TCAs |
| Pain fiber C or A delta | Calcium and sodium channel | Channel blockers |
| Interneuron | NMDA,GABA, and endogenous opioids | Magnesium, PCP, GABA, and opioids |
| Reticular formation, thalamus, mesencephalon, and hypothalamus | GABA, norepinephrine, substance P, serotonin, and opioids | GABA, norepinephrine, β-blocker, capsaicin, serotonin, and opioids |
| Frontal cortex, insular cortex, limbic structures, and sensory cortex | GABA, norepinephrine, substance P, serotonin, and opioids | GABA, norepinephrine, β-blocker, capsaicin, serotonin, opioids, AEDs, TCAs, benzodiazepines, and barbiturates |
AEDs, antiepileptics; ASA, acetylsalicylic acid (aspirin); GABA, γ-aminobutyric acid; NMDA, N-methyl-D-aspartate; NSAIDs, nonsteroidal anti-inflammatory drugs; PCP, phencyclidine hydrochloride; TCAs, tricyclic antidepressants.
The characteristics of the pain also play a role in the treatment. It is important to obtain the following characteristics of the pain: its quality, location, intensity, duration, periodicity, exacerbating and relieving factors, present and past pain management, and associated signs and symptoms.
Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage. Therefore, assessment of pain requires an examination of the area of pain referral and any other diagnostic tests that will assist in determining the appropriate treatment.
Often only pain intensity is considered in the pain history; by itself, however, it may lead to unnecessary treatments. When there are difficulties in communication such as may occur in the very young, the very old, the mildly confused, and patients of different language, culture, or background, a pain assessment rating scale may be the only information available. Most widely used “pain scales” are based on 10 divisions. Because it is necessary to follow pain trends over time and with treatment, often between varying health care workers and health facilities, only a 10-division scale should be used until other scales become universal.9,10
A numeric rating scale asks the patient or the health care worker to rate the pain on a scale of 0 to 10, with 0 being no pain at all and 10 being the worst possible pain the patient or health care worker could imagine. Often patients will state that they have a high pain threshold, much higher than others. However, it must be emphasized to patients that the worst possible pain that they could imagine is a 10, and the current pain is in relation to that level, not based on others’ perception. It may be appropriate at times to ask women who have had children by vaginal delivery, as ascertained in the history, to compare the current pain to the pain experienced during labor, which may have been a 10 (Fig. 12–1).
The modified Visual Analog Scale is a 10 cm line marked from 0 to 10, with 0 being no pain at all and 10 being the worst possible pain the patient could imagine. When teamed with the commonly used scale such as the American Cancer Society, Facial Pain Scale or Wong-Baker Faces Rating Scale for Children, which consists of six or eight images of faces with varying expressions, from a smiling face (0) to a crying face (10), the Visual Analog Scale becomes the ultimate scale for the awake person (Fig. 12–2).
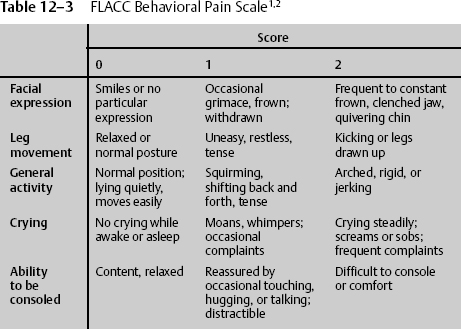
Severely debilitated patients and those with severe head injuries are physically not able to use these scales to indicate their level of pain. In these cases, health care workers are responsible for acting in the best interest of the individual patient to relieve suffering without causing untoward side effects. A behavioral pain scale is useful when patients cannot communicate. One of the most widely accepted behavioral pain scales is the 10-point FLACC scale. The patient is assessed in five categories and given 0, 1, or 2 points depending on the behavior being demonstrated. The behaviors are facial expression, leg movement, general activity, crying, and ability to be consoled (Table 12–3). The FLACC scale can also be used with children ages 6 months to 3 years.
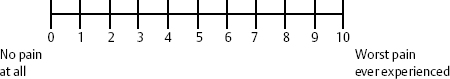
Figure 12–1 Commonly used pain scale. Patient points to number on scale after health-care worker describes “0” as no pain at all and “10” as worst pain ever experienced.
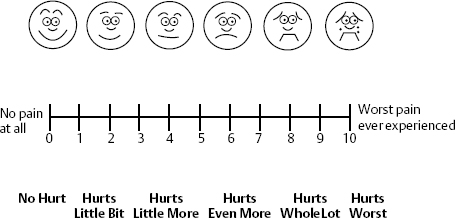
Figure 12–2 Visual Analog Scale (VAS) and Wong-Baker Faces Scale allows additional clues for patients to adequately assess their pain. The health-care worker describes “0” as no pain at all and “10” as worst pain ever experienced. The faces help the patient decide how he or she currently feels.
 Management of Pain
Management of Pain
Nonsteroidal Anti-inflammatory Drugs
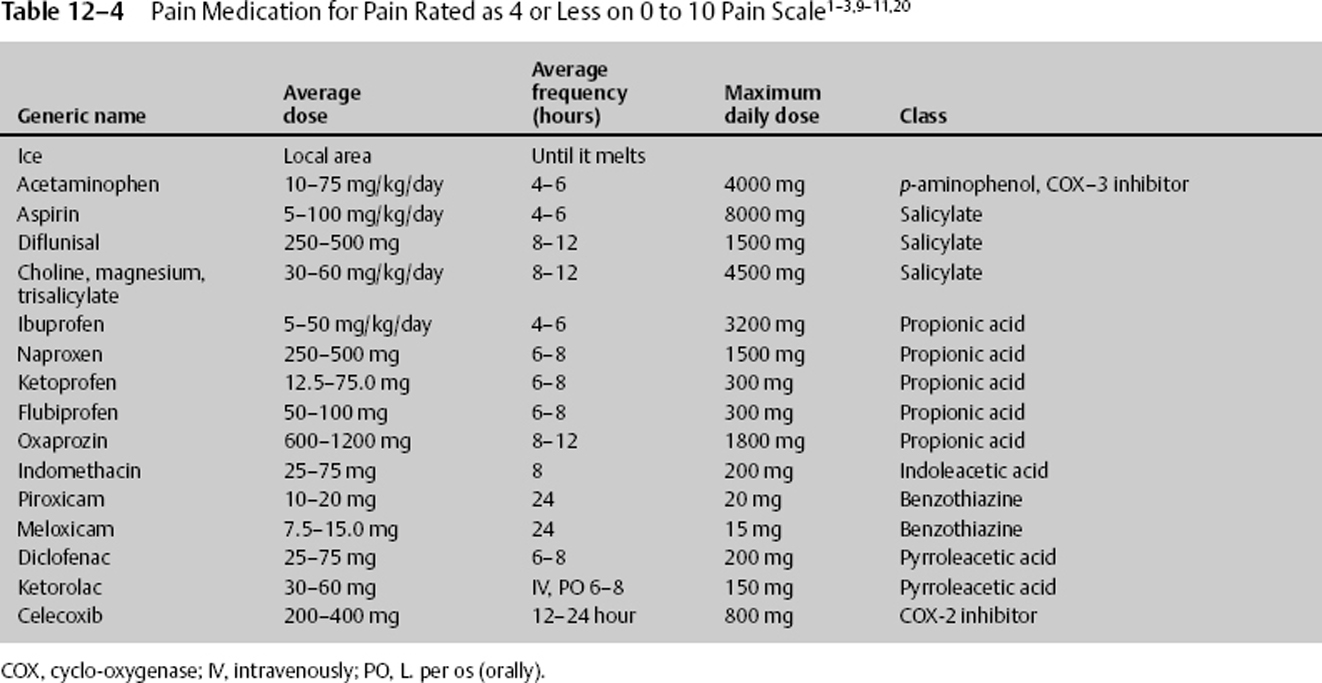
Once a patient’s pain can be assessed using a pain scale, medication and other treatment can be quickly entertained. Pain that is rated as 4 or less can be managed with ice, acetaminophen, or nonsteroidal anti-inflammatory drugs (NSAIDs), with the last two inhibiting an isoform of the enzyme cyclo-oxygenase (COX 1–3) blocking prostaglandin synthesis.11 The usual dose for acetaminophen (N-acetyl-p-aminophenol [APAP]) is 10 to 75 mg/kg/day in 4 to 6 divided doses. The maximum adult daily dose is 4000 mg. It is now believed that an initial large dose of 50 mg/kg can be given, yielding relief comparable to that of some narcotics while maintaining a high safety profile. The side effects of acetaminophen are seen with elevated multiple doses, although they can occur with a single dose. They are acute hepatic necrosis, or liver toxicity nephrotoxicity and, with chronic use, thrombocytopenia. The manufacturers of acetaminophen have voluntarily upgraded warnings that large doses can cause irreversible liver failure. Those with impaired liver function or regular alcohol use should take no more than 2 g daily. Anyone who takes more than three alcoholic drinks a day should be advised to avoid acetaminophen.
NSAIDs should be withheld for 4 to 10 days after mild bleeding and not given for at least 4 to 10 days after severe bleeding. NSAIDs inhibit platelet function only while at therapeutic dosage. They should be given with a stomach protectant and adequate hydration to help prevent side effects. The usual dose for ibuprofen is to 50 mg/kg divided into 4 to 6 doses per day.
Ketorolac is another anti-inflammatory drug that can be given intravenously or orally. The usual dose for ketorolac is 30 to 60 mg per dose and can be given every 6 to 8 hours. Ketorolac can be given immediately after such neurosurgical procedures as lumbar diskectomy and lumbar spinal fusion when bleeding has been controlled. During these procedures, ketorolac 30 mg IV may be given initially, followed by 120 mg in 500 cc saline at 10 cc/hour continuous drip until completed. This treatment modality, along with lidocaine patches or infusion, may greatly decrease or obviate the need for narcotics.
NSAIDs belong to multiple drug classes, with each class affecting individuals differently. Therefore, if one class of NSAID or even a drug in the same class does not work, consider treatment with another (Table 12–4).
NSAID Side Effects
None of the drugs in this group should be given if there is a known sensitivity to an NSAID. Salicylates should not be given to children with viral infections. In general, the nonselective COX inhibitor NSAIDs have similar side effects, such as dyspepsia; ulcers; GI perforation and bleeding (antiplatelet activity lasts for 2–3 days while therapeutic); kidney and liver dysfunction; hypersensitivity reaction, such as urticaria-angioedema; respiratory, attention, and memory deficits; headache; and tinnitus. Rofecoxib, a COX-2 inhibitor, was recently shown to have cardiac side effects, such as MI and stroke, and has been voluntarily removed from the market. Celecoxib, another COX-2 inhibitor, has not been removed from the market. Celecoxib side effects are thought to be less problematic than other NSAIDs.
There is no significant difference between the GI effects of nonselective COX NSAIDs and a proton pump inhibitor and selective COX-2 NSAIDs. ASA inhibits platelet function irreversibly, whereas NSAIDs inhibit platelet function only while at therapeutic dosage. Furthermore, certain nonselective COX inhibitors, such as ibuprofen, will block the antiplatelet of ASA, but others, such as naproxen, will not.
Other Pain Treatment
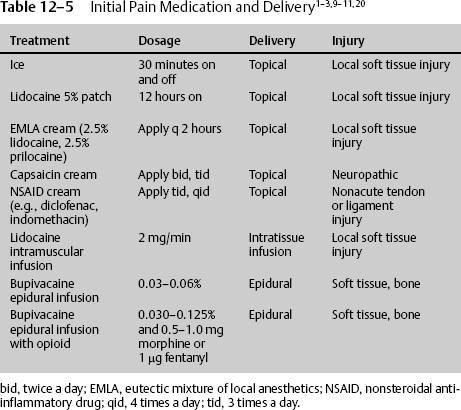
There are numerous ways to administer effective pain treatment, and in the neurologic patient, these routes must be tried unless there is a contraindication. Initially, local pain should be treated with ice. Icing works by stimulating large A delta fibers in the peripheral nervous system, increasing output above C fibers, and inhibiting pain transmission. Longer lasting pain, such as that caused by muscle spasm, may be treated with ice, heat, or both, depending on the response. If there is severe extremity or axial pain epidural at the approximate dermatome or caudal, anesthetics and analgesics may be delivered. The elevated pain from the disruption or destruction of large muscle areas may be treated appropriately with the local continuous infusion of long-or short-acting lidocaine derivatives. When the pain is of less severity, or to augment delivery of other pain medication, topical solutions can be utilized. Although used for chronic and neuropathic pain, they can be used for acute pain. Compounds such as a 5% lidocaine patch can significantly reduce pain and improve the quality of life. Lidocaine is believed to block sodium channels in the damaged nerve endings. It can be applied to the newly traumatized or operated tissue. The 5% lidocaine patch should not be applied directly to the open tissue or freshly sutured tissue. In those cases, it can be applied on both sides of the wound for 12 continuous hours per day (Table 12–5).
Nonopioid Pain Treatment
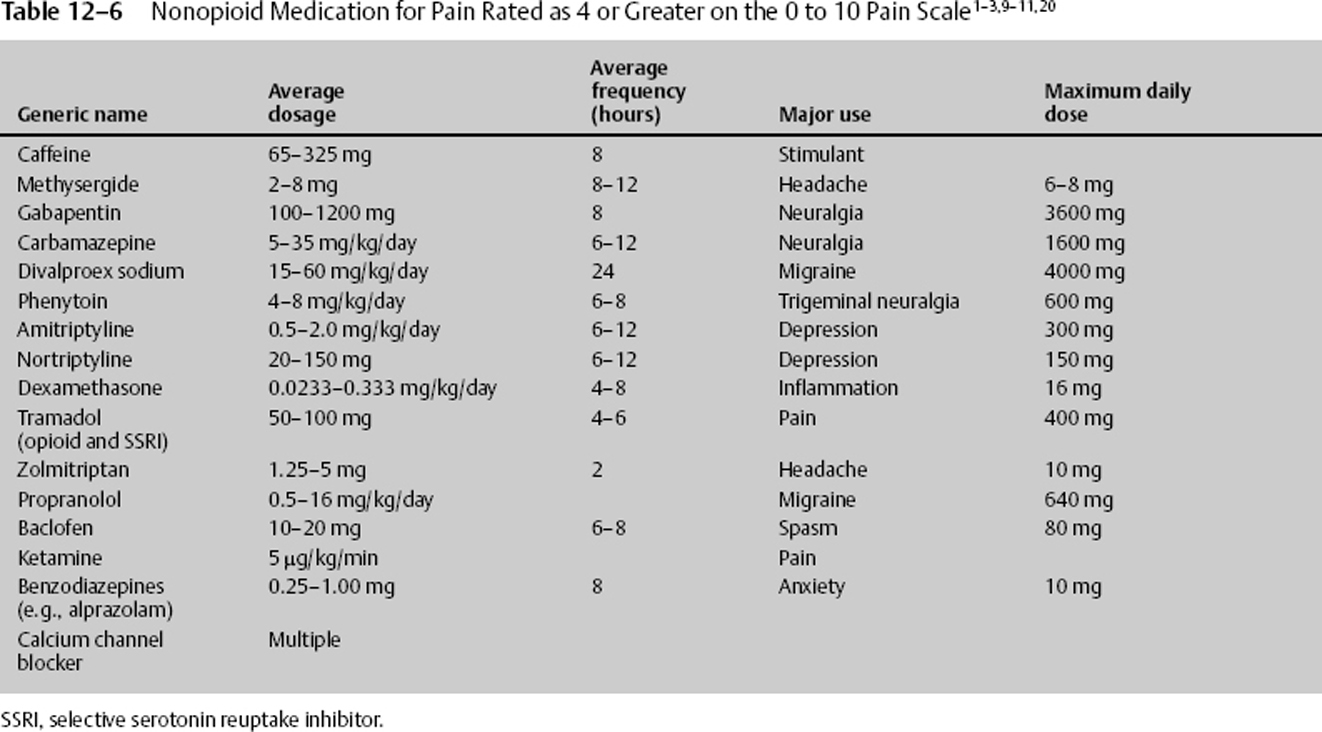
For pain greater than 4 on a 0 to 10 pain scale, acetaminophen and NSAID, as well as other nonconventional, nonnarcotic complementary drugs, can be used (Table 12–6). Each may be used with narcotics, potentiating the effects of a smaller narcotic dosage, resulting in less sedation and other deleterious side effects. The nonconventional medications can be benefited from, especially if the history can provide clues as to environmental, physiologic, or psychological factors influencing the current pain. The nonconventional medications include antiepileptic drugs (AEDs), tricyclic antidepressants (TCAs), GABA, norepinephrine, α- and β-blockers, capsaicin, serotonin, benzodiazepines, caffeine, and barbiturates. These nonconventional medications are particularly beneficial in neuropathic and chronic pain.
Nonconventional pain medications work in multiple areas and by a variety of mechanisms, as listed here.
- TCAs inhibit neuronal discharge, decrease sensitivity of adrenergic receptors, block reuptake of norepinephrine and serotonin, and bind to histaminergic, cholinergic, and adrenergic receptors.
- Selective serotonin reuptake inhibitors (SSRIs) potentiate serotonin and norepinephrine pathways, inhibit cytochrome P-450, and assist in treating patients with depression and anxiety. Side effects include tremor, fever, diarrhea, delirium, and increased muscle tone.
- AEDs suppress abnormal neuronal discharges by blocking sodium channels, whereas others increase the inhibiting GABA transmission.
- Benzodiazepines potentiate inhibitory GABA transmission while also having antianxiety and antispasticity properties.
- Alpha-adrenergic blockers decrease hyperarousal symptoms by activating autoinhibitory presynaptic receptors of the locus cerulus.
Common potentiating adjuvant nonnarcotic pain medications are listed in Table 12–6. The medication should be carefully chosen and reflect the clinical situation associated with the drug’s major use. For example, if depression exacerbates the pain, consider the addition of amitriptyline.
Opioid Pain Treatment
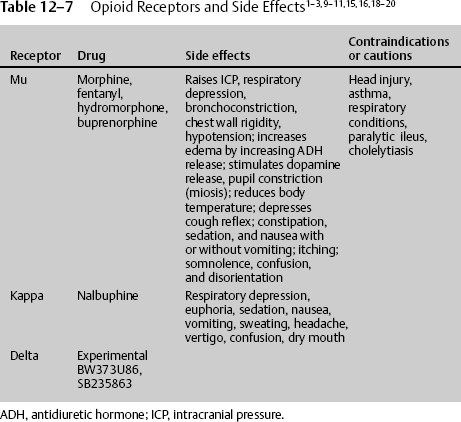
The most commonly prescribed medications for severe pain are opioids. Theophrastus first referenced opium in the third century BC; now there are four classes of opioids, representing the particular receptor they stimulate: mu, kappa, delta, and the newest, nociceptin/orphanin FG (N/OFQ).7 Mu was named for the prototype agonist morphine. It is stimulated by endogenous enkephalins and β-endorphins. Known drugs are morphine, buprenorphine, fentanyl, meperidine, and methadone. Kappa was named for the prototype ketocyclazocine. It is stimulated by endogenous dynorphins. Known drugs are butorphanol, nalbuphine, and pentazocine. Delta was discovered in the mouse vas deferens. It is stimulated by endogenous enkephalins and β-endorphins, which also bind to the mu receptor. There are no known clinical drugs, although experimental drugs exist.
Multiple investigators believe in the beneficial role of opioids. Faden12 suggests that by utilizing selective opioids, novel approaches to the treatment of CNS injury can be developed. Among the subtypes of opioid receptors, mu and delta receptors either inhibit or potentiate NMDA receptor-mediated events, whereas kappa opioids antagonize NMDA receptor-mediated activity, providing more neurotoxic protection. To assist healing during times of nervous system injury, the body’s opioid systems have both a neurodestructive and a neuroprotective role. Mu and kappa-1 subtype receptors appear to mediate neuroprotective actions, and kappa-2 receptors are involved in secondary injury responses. Dynorphin, an endogenous kappa receptor stimulator, has marked neurotoxic effects when given intrathecally at subinjury doses to rats, exacerbating the response to brain or spinal cord trauma,12 probably by stimulating a kappa receptor subtype.
Using animal models, other selective opiate receptor antagonists, such as naloxone, and the endogenous opiate antagonist thyrotropin-releasing hormone (TRH) have improved outcome and physiological variables following hypovolemia, spinal cord trauma, and head injury. High-dose naloxone in the mg/kg range improves blood pressure, neurologic motor function, outcome, and survival.13,14 To take full advantage of the effects opioids have on individual receptor stimulators and because humans experience the clinical effects of opioids differently, specific receptor stimulators and inhibitors must continue to be investigated and understood.
Kappa Opioid Receptor Stimulators
Opioid receptors are classified into four types: mu, delta, kappa, and N/OFQ. Among these types, the kappa receptor system has become most attractive. According to Ikeda and Matsumoto,15 by targeting the kappa receptor, novel therapeutic agents beyond pain and its relief can potentially be developed. Endogenous opioid systems have been proposed as secondary or delayed brain injury factors, largely on the basis of the therapeutic efficacy of opioid receptor antagonists or agonists. Ikeda and Matsumoto15 demonstrated that the novel kappa opioid agonist RU51599 has a neuroprotective effect on traumatic and ischemic brain edema, while also having an analgesic effect. Hudson et al16 demonstrated the neuroprotective effects of the kappa opioid–related anticonvulsants U-50488H and U-54494A in a neurotoxic model of NMDA-induced brain injury in the neonatal rat. Tortella and DeCoster17 found that kappa receptor opioids, specifically the arylacetamide series of kappa opioid analgesics, are novel pharmacotherapeutic treatments for epilepsy, stroke, or trauma-related brain or spinal cord injury. The kappa opioid shares common properties with all opioids, being neuroprotective but, unlike mu receptors, acting directly in the brain to reduce ICP by inhibiting antidiuretic hormone (arginine vasopressin [AVP]) secretion and promoting water excretion in humans.18 AVP regulates brain water content and is elevated in the cerebrospinal fluid of patients with ischemia and traumatic brain injuries. Ikeda et al19 demonstrated the protective effect of RU51599, a selective kappa opioid agonist and AVP release inhibitor, thereby improving brain edema. Kappa receptor stimulation increases diuresis, reducing edema and increasing ICP, leading to increases in cerebral perfusion pressure. Kappa receptor stimulators cause respiratory depression but less than mu receptor stimulation. They also cause dysphoria, which can be problematic.
Nalbuphine is a potent kappa agonist and mu antagonist analgesic with a low side effect profile and low abuse potential, possibly because it inhibits midbrain dopamine release. Because it is a mu antagonist, it should be used with caution in those individuals who are addicted to mu receptor stimulators, such as morphine, because it can cause a withdrawal problem.
Mu Opioid Receptor Stimulators
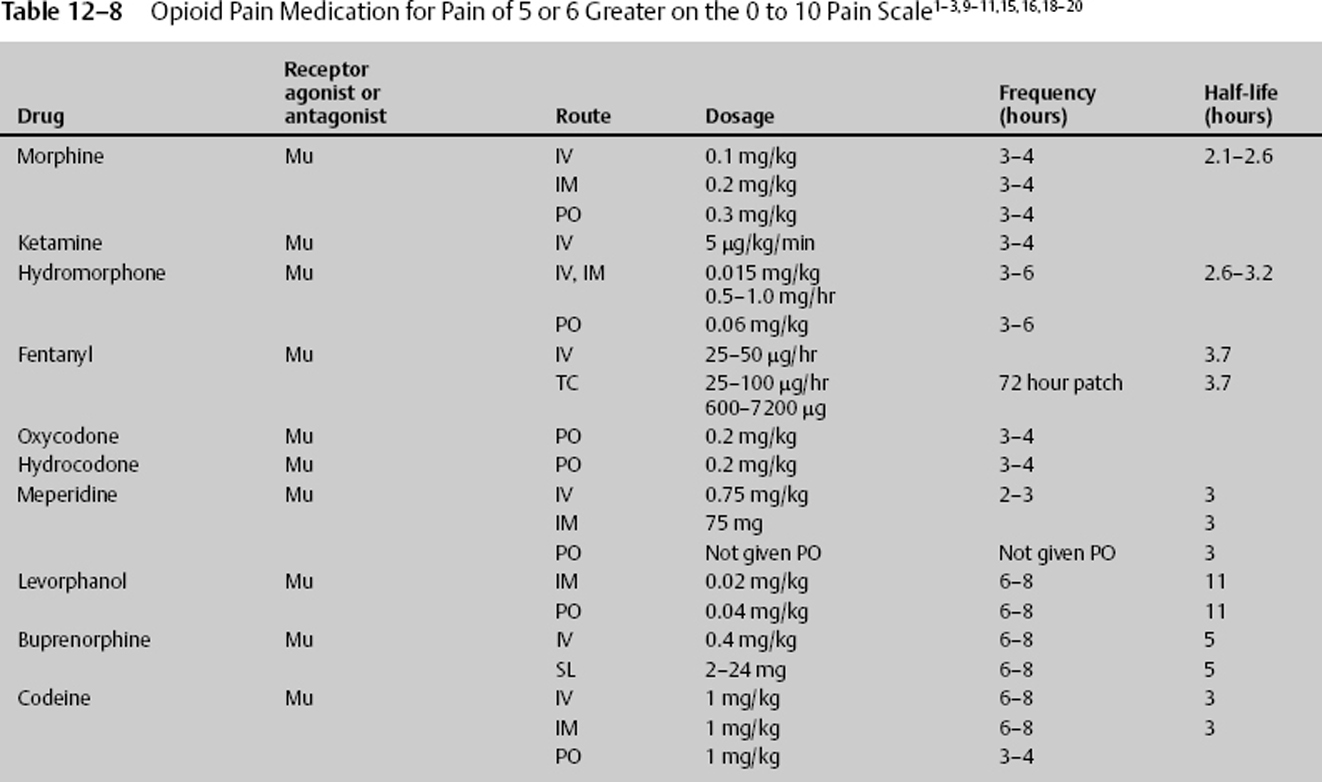
The prototypical mu receptor stimulator is morphine, which has many benefits. In neurologic disease, it is neuroprotective to the cell. Morphine may also be used for preoperative sedation and dyspnea associated with acute left ventricular failure and pulmonary edema. Camphorated tincture of opium, another mu receptor stimulator, can treat severe diarrhea and intestinal cramping, and codeine is helpful with severe persistent cough. At times when very mild hyperventilation may be used, such as after head injury, morphine blunts the reflex vasoconstriction caused by decreased pCO2 and decreases peripheral vascular resistance.20
The potential adverse effects of ketamine in neurosurgical anesthesia have been well established. The side effects of this mu receptor stimulator are increased ICP and cerebral blood flow (CBF). De Nadal et al21,22 demonstrated in human brain trauma subjects that when carbon dioxide reactivity was preserved, 56.7% of the patients showed impaired or abolished autoregulation to hypertensive challenge. In both groups after mu receptor stimulation with either morphine or fentanyl, there was a significant increase in ICP and a decrease in mean arterial blood pressure and cerebral perfusion pressure, but arterial-venous difference of oxygen (AVDO2) estimated CBF remain unchanged. In patients with preserved carbon dioxide reactivity, the opioid-induced ICP increase was even greater. This demonstrated the vasodilating effects of fentanyl. In additional studies, de Nadal and others came to the conclusion that potent opioids cause greater increases of ICP, due to methods other than activation of the vasodilator cascade. Sperry et al23 showed that mu receptor stimulators are associated with a statistically significant ICP increase of 8.2 mm Hg for fentanyl, and an increase of 6.1 mm Hg for sufentanil. Both drugs caused statistically significant decreases in mean arterial blood pressure (MAP): fentanyl, 11.6 mm Hg; sufentanil, 10.5 mm Hg. No significant changes in heart rate occurred. Their results indicate that modest doses of potent opioids can significantly increase ICP in patients with severe head trauma.23 Other investigators proved the same, that sufentanil, fentanyl, and alfentanil infusions cause a significant but transient increase in ICP accompanied by a significant decrease in MAP.24 Hydromorphone, a mu receptor agonist, also significantly increased regional CBF in the anterior cingulate cortex, both amygdalae, and the thalamus, all structures belonging to the limbic system. Not all mu receptor stimulators cause cerebral vasodilation.
Mixed Receptor Stimulators
Butorphanol, a kappa agonist/mu antagonist, causes a less distinct picture of regional CBF increases, mainly in the area of both temporal lobes. The same mu receptor antagonist shows improvement in outcome after experimental brain trauma by the actions of kappa-opioid receptor agonists, affecting cellular bioenergetics.25
In addition to vasodilation, mu receptor stimulators produce mainly euphoria, whereas kappa agonists are more likely to produce dysphoria. The administrations of morphine and pentazocine increased dopamine turnover in the striatum and hypothalamus in a drug dose–dependent manner. The stimulative effects of butorphanol on the dopamine system were weaker than those of morphine and pentazocine, and there were no dose dependencies in the effects of butorphanol. Butorphanol, morphine, and pentazocine increased 5-hydroxytryptamine (5-HT, serotonin) turnover. It has been hypothesized that the nucleus paragigantocellularis (PGi) plays an important role in the development of physical dependence on opioids, including the prototype mu-opi-oid receptor agonist morphine and the mixed agonist/antagonist butorphanol, which shows selective kappa-opioid receptor agonist activity.
Opioids and Their Side Effects
Just as NSAIDs belong to different groups, with considerable variability in efficacy, opioid groups vary in their ability to treat pain, even in the same individual. If one opioid does not work, consider treatment with another one. The first and most widely used opioid is the group that stimulates the mu receptor; these include morphine, Demerol, Dilaudid, and fentanyl. The side effects of this group share some commonality with most opioid receptor stimulators and, as expected, demonstrate unique risks. Mu receptor stimulators cause increased ICP, both directly and indirectly, by vasodilatory and nonvasodilatory ways;7,9,10,20,26 respiratory depression; bronchoconstriction; chest wall rigidity; hypotension; increased edema by increasing antidiuretic hormone (ADH) release; stimulated dopamine release; constipation; reduced body temperature; sedation; nausea with or without vomiting; itching; somnolence; confusion and disorientation; hallucinations; and seizures at high doses. The mu acting drug also stimulates dopamine release, leading to addiction.
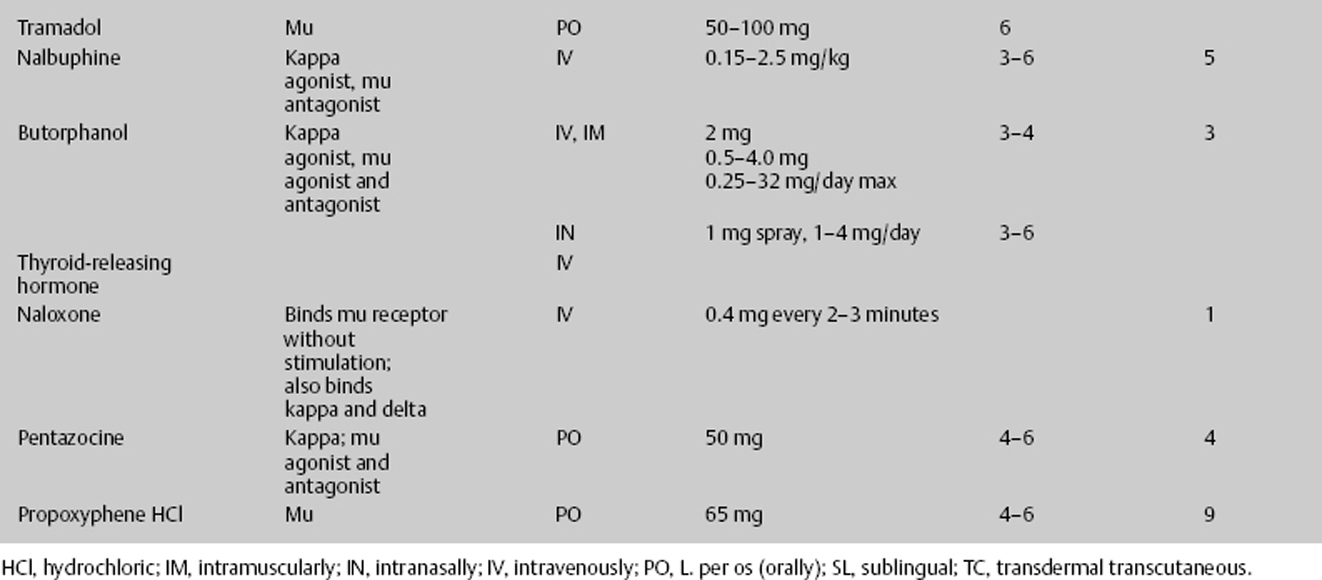
Therefore, when prescribing a high-dose intravenous opioid or opioid infusion, vital signs must be closely monitored. There should be continuous pulse oximetry, and the physician should be notified for decreased oxygenation. Blood pressure and respirations should be recorded every 15 minutes for 2 hours, then every 1 hour for 4 hours, then every 4 hours thereafter. With each increase in dosage, the vital sequence should restart. The medication should be stopped and the physician notified if the respiratory rate is less than 10 per minute or the systolic blood pressure (SBP) is less than 90 mm Hg. Antidotes for opioids must be immediately available. Naloxone 1 mg ampule should be on the patient’s floor. If the respiratory rate is less than 8 per minute, give naloxone 0.2 mg intravenous push (IVP) and call the attending physician; the dose may be repeated in 5 minutes. For itching, have available diphenhydramine 25 mg IV/PO every 6 hours. For initial nausea and vomiting, which usually resolve with the second dose, have available an antiemetic such as granisetron 10 μg/kg IV over 5 minutes every 4 hours or comparable agent. Furthermore, when discontinuing patient-controlled analgesia (PCA) if greater than 3 days of continuous infusion, wean the medication (Table 12–7).
- Ten percent of the population lacks the enzyme needed to activate codeine. As a desired treatment, codeine may cause nausea and constipation.
- Tramodol lowers the seizure threshold.
- Fentanyl transdermal takes 12 to 16 hours to produce a therapeutic effect and 48 hours to reach steady state.
- Seriously consider the use of meperidine in the neurosurgical intensive care unit (NICU) patient because of neurotoxic risks of anxiety, tremors, myoclonus, and seizures.
- Give a loading dose of the narcotic, then an hourly dose of approximately 1/6 the loading dose.
- Most opiates are cleared by renal and hepatic means (Table 12–8).
 General Considerations in Treating Pain
General Considerations in Treating Pain
Treat pain early, when it begins; do not wait until pain is out of control. When pain becomes severe, it will require a much greater dosage. Consider providing medication around the clock when there is an initial injury, as opposed to giving medication only when asked for by the patient or only when the patient appears symptomatic. There will also be times when there is breakthrough pain. This pain is usually of moderate to severe intensity, occurs rapidly, usually in less than 3 minutes, is of relatively short duration, and occurs 1 to 4 times per day. Therefore, different medications should be prescribed for initial treatment and recurring pain. It is even beneficial to prescribe patient-controlled analgesia or continuous intravenous infusion of pain medication (Table 12–9).
In addition to cautiously looking for reasons to decrease or discontinue the medication, NICU personnel must document the medication’s efficacy. The attending physician must be notified if the pain remains greater than 4 on a 1–10 pain scale for 3 consecutive hours. If there is breakthrough pain, acetaminophen, 650 mg or more, can be given every 4 to 6 hours. Consider additional treatment should a pain pump or medication stop being available.
| Scale score | Treatment |
| 0–4 | Ice + APAP; possibly low-dose NSAIDs, possibly topical |
| 5–9 | Ice + APAP + ketorolac; possibly high-dose NSAIDs, low-dose opioids |
| 10 | Ice + APAP + ketorolac; possibly high-dose NSAIDs, high-dose opioids |
APAP, acetaminophfen; NSAIDs, nonsteroidal anti-inflammatory drugs.
< div class='tao-gold-member'>