3. Indications for intervention
a. Both the decision to intervene and the manner of intervention are dependent upon the degree of impairment the patient is experiencing as well as the future hazards posed by the AVM and the plan of intervention. Therapeutic plans must have a reasonable chance of success in eradicating the lesion or to cure or stabilize deficits.
b. Beyond the dangers of a particular therapy there is evidence within the literature that incompletely treated AVMs and intervention itself may increase risk of rupture [8]. Posited mechanisms include detrimental alteration of the hemodynamics within the AVM and loss of fragile vascular integrity as an ischemic nidus necroses or attempts anastomosis with immature vasculature recruited through angiogenesis.
c. Again the chance of hemorrhage mostly dictates the need for intervention. The risk of hemorrhage without therapy must be higher than that posed by undergoing the therapy. This can be estimated by aspects of the AVM identified through radiologic assessment that are associated with hemorrhage as described previously.
d. Special populations include children, in whom intervention is typically pursued; the elderly, in whom intervention is less aggressive; and pregnant patients, in whom intervention depends upon the assessment of hemorrhage risk for a particular patient versus risks to the fetus [5].
e. Other plausible indications for intervention include intractable seizures and impaired psychomotor function from ischemia or mass effect, cardiovascular failure from severe shunting, and quality of life issues such as anticipated childbirth.
D. Management
1. Clinical considerations
a. Detailing the intensive care of ruptured AVMs is part of the consideration of intracerebral hemorrhage therapy in general and will not be highlighted here apart from the radiologic assessment and craniotomy for life-threatening hematoma evacuation as discussed. Insight into the controversial points of deliberation concerning the nonemergent, subsequent management of AVMs including the advantages and liabilities of treatment courses will instead be evaluated (Tables 10.2 and 10.3).
4
b. Contemporary AVM treatment is multimodal and founded upon a coordinated multidisciplinary team approach including medical professionals in intensive medicine, neurology, neurosurgery, and radiology (Fig. 10.1A–C). There are four treatment options: observation, surgery, neuroendovascular intervention, and radiosurgery.
5
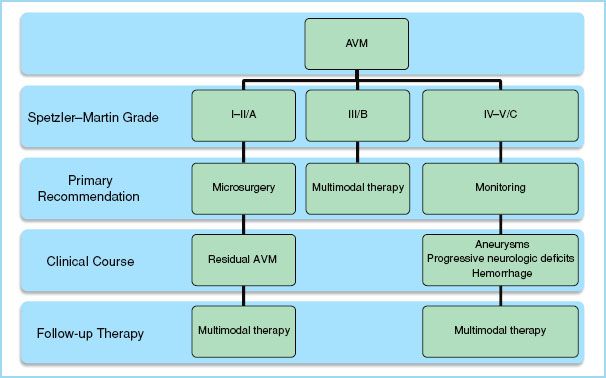
c. Upon full assessment of prospective management strategies with respect to a particular patient’s concept of personal integrity, psychological well-being, quality of life, and life expectancy, patients should be advised of risk management during their clinical course. This may entail a sequential intervention where a less definitive therapy is initially pursued due to its lower risk. This acts as a catalyst by lowering the otherwise unacceptable risk of a more definitive therapy to succeed it. For example, an interventional neuroradiologist may embolize feeding arteries to an AVM which are situated in deep, eloquent areas of the brain preoperatively in order to facilitate a less traumatic and hemorrhagic operation. The overall risk of the entire treatment plan is therefore reduced through the employment of a catalytic therapy initially. The treatment plan of a patient is determined through application of the Spetzler–Martin scale (Table 10.4) which recently has proposed revisions (Table 10.5). Each major therapeutic modality will be highlighted within a cumulative algorithm (Fig. 10.2).
CLINICAL PEARL
The definitive procedure can be somewhat temporized until the patient stabilizes.

FIGURE 10.1 A–C: A 41-year-old male presented to emergency room after a generalized seizure. Subsequent investigations revealed large temporoparietal AVM. Patient underwent successful two-stage embolization (afferents of middle cerebral artery and then of anterior cerebral artery) followed by radiosurgery. (Courtesy of John Whapham, M.D., Assistant Professor, Director Neurointerventional Program, Loyola University Medical Center.)
2. Monitoring
a. The most conservative care plan is one of monitoring with serial neurologic and radiologic evaluations including CT and MRI. This would seem to be most prudent for asymptomatic patients but can be utilized even for patients with declared AVMs who, following assessment, are estimated to be at greater risk for morbidity and mortality from intervention.
b. There are few prospective trials comparing treatment to monitoring for unruptured AVMs but one expected study finalizing in 2017 is a randomized trial of unruptured brain AVMs (ARUBA) [9].
c. While “to do no harm” in the spirit of the Hippocratic oath and potentially less cost are the most obvious benefits, the specter of hemorrhage is the most ominous risk of monitoring.
3. Microsurgery
a. This can be utilized as monotherapy with permissive AVM anatomy and location or as a succeeding therapy in a sequential intervention.
b. The intraoperative course involves dissection of arterial vasculature on course to the lesion with temporary clipping to maintain hemostasis, definitive transection of feeding arteries as they are identified, dissection of the nidus, and dissection and transection of venous drainage.
c. Operative management has the primary advantage of excising AVMs at once that either cannot be treated effectively by embolization or radiosurgery or can only be treated by these means in stages.
d. Surgical patients tend to have longer hospital courses, can have higher rates of neurologic dysfunction, and are under threat from normal perfusion pressure hemorrhage in the acute postoperative period.
4. Neuroendovascular embolization
a. First, this can be used as monotherapy to obliterate a small AVM in its entirety at once or in a staged procedure with a larger lesion to avoid acute destabilization of the AVM’s hemodynamics leading to rupture.
b. Second, as a catalyst for neurosurgery or radiosurgery. Compartments of the nidus, aneurysms of the nidus or feeding arteries, and feeding arteries deemed operatively inaccessible are targeted. Also, often in conjunction with coiling, high-flow nidal fistulas can be preoperatively diminished.
c. Lastly, partial embolization can be used as palliative therapy for debilitating symptoms from large, inoperable AVMs due to intracerebral edema or shunt. This is usually not permanent as the residual nidus, now ischemic, will act to revascularize and reconstitute itself [10].
CLINICAL PEARL
Partially obliterated AVMs can reconstitute and are at higher risk of rupture.
d. Once arterial access has been obtained, routinely through the femoral vasculature but also via the brachial or carotid vasculature as necessary, the cerebral circulation is acquired by either of two delivery systems: Smaller, flexible, flow-directed microcatheters; or larger, more rigid catheters directed over less-compliant guidewires. The latter system assumes an increased risk of vascular trauma and perforation.
e. Two liquid polymer embolic substances used are n-butyl cyanoacrylate (commercially known as Trufill®) and ethylene-vinyl alcohol (commercially known as Onyx®) which act to acutely obliterate the vascular lumen while initiating an inflammatory reaction toward fibrosis and induration chronically.
f. Factors such as solution viscosity, polymerization rate, and injection rate can be chosen toward optimal penetration of the nidus while minimizing the possibility of extension to the venous system with threatened pulmonary embolization or reflux of these compounds out of the AVM to the arterial circulation with unintentional embolization of perinidal neural sites. The premature occlusion of venous drainage should also be avoided to prevent distension of the AVM with impending rupture [10].
g. Primarily, use of the cyanoacrylate polymer is advantageous in that it is administered through the compliant flow-directed microcatheter. However, with extended injection times the threat of catheter retention increases because this substance is adhesive and risks bonding to the catheter within the vasculature.
h. While the ethylene-vinyl alcohol product is not adhesive and can facilitate a more controlled, measured, and extensive embolization, it is deployed using catheters over guidewires.
i. Coils also can be delivered by either system with the particular advantage of reducing flow through intranidal fistulas so that liquid polymers can subsequently be employed under reduced risk of crossing into venous outflow.
j. There is no formal consensus as to whether deep sedation or general anesthesia with complete muscle relaxation is best for patients undergoing endovascular therapy, but logic directs that general anesthesia would at least be more appropriate for the use of wire-guided systems to obviate the hazard of vascular perforation from patient motion.
k. Overall, neuroendovascular interventions decrease the time of hospitalization for patients but have limits to the size of the lesions that are treatable.
5. Radiosurgery
a. Radiation therapy can be considered as monotherapy in the treatment of small lesions that are not candidates for other methods and in Spetzler–Martin grade three or greater lesions with the goal of cure rather than palliation.
b. Radiosurgery is noninvasive, does not require lengthy hospitalization, and can be combined with embolization and or microsurgery. While there are some reports that prior embolization reduces the efficacy of radiosurgery, it is possible that all three techniques are employed in a scenario such as embolization prior to microsurgery with radiotherapy for any residual lesion. By definition however, radiosurgery is a staged process that can take up to 3 years to obliterate the AVM during which time there is continued risk for rupture. In addition, intracerebral edema, necrosis, cyst formation, and malignant tumors can result from this technique.
E. Radiation safety. As anesthesiology practitioners are increasingly consulted for patient care in interventional radiologic procedures, it should be remembered that precautions against radiation exposure should be maintained just as universal precautions. Protective eyewear, thyroid shields, and gowns should be worn as well as a dosimeter to monitor the cumulative exposure with extensive duty in a radiology suite. Using a mobile shield or exiting the room entirely during periods of intense radiation should be utilized.
II. Anesthetic management
A. General considerations
1. The indications, timing, and succession for various interventions are still in flux [9] but overall the anesthesiologist can encounter AVMs in the interventional radiology suite, in the operating room, or in the radiosurgery center. We elected to present their management together because in the modern era of the “hybrid operating room” the physiologic principles are the main guide to the management independent of the place of intervention.
2. The procedures are done on an elective or emergent basis. Fortunately, AVMs have a lower chance of rebleeding, so the definitive procedure can be somewhat temporized until the patient stabilizes. A common indication for emergent treatment is decompression of a large hematoma after an initial bleed. The caveats of emergent cases are well known to any practicing anesthesiologist. We recommend establishing institutional-based protocols and clinical pathways ahead of time that delineate various team member responsibilities. We should remember that often speed is essential. In cases of neurologic deterioration, either spontaneous or iatrogenic, prompt evacuation of hematoma (within 30 minutes if possible) is associated with better outcomes [11].
3. Anesthesiologists need to have a deep understanding of the current and rapidly changing physiopathologic principles that govern the interactions between AVMs and the surrounding brain. We can be called to action to either strictly enforce normal homeostatic variables and/or create controlled disturbances intended to facilitate the operative correction and/or to compensate for various abnormalities present or iatrogenically created.
6
4. The general principles of neuroanesthesia apply to the management of AVMs (Table 10.6). We will especially try to make recommendations following an evidence-based approach.
Table 10.6 Common neurophysiologic targets and neuroanesthetic interventions

5. Providing anesthesia care in the interventional neuroradiology or radiosurgery suites invokes the general precautions necessary at remote locations, including but not limited to the unavailability of specialized help in case of emergency, decreased lighting, decreased mobility of the operating table, unusual configurations for the position of the anesthesia machine and cart, decreased direct access to the patient (increased distance and interposition of imaging tools).
B. Preoperative considerations
1. Most of preoperative anesthetic considerations are common to all surgical patients, however the presence of specific comorbidities should be actively sought.
2. All patients should have a preoperative anesthesia evaluation performed before the beginning of the procedure, including all emergent and monitored anesthesia care cases:
a. Cardiac history, including the presence of coronary disease and myocardium at risk, heart systolic or diastolic dysfunction (reports of ejection fraction percentage), intracardiac shunts (for sitting position procedures), and neurogenic pulmonary edema. Recent cardiac stents are associated with a higher risk of in-stent thrombosis if antiplatelet medications are to be stopped prematurely. Risks and benefits of open versus neuroradiologic procedures should be analyzed carefully in these cases.
b. Arterial hypertensive history predisposes the patient to wider fluctuations in blood pressure. While these are generally of reduced significance in the general surgical population, it might be different for the patient with an AVM that has regions of the brain chronically exposed to disparities in perfusion. However, in population studies, the relationship between hypertension and AVM progression and hemorrhage is not proven definitively.
c. Lung diseases. Chronic obstructive pulmonary disease or asthma can influence our ability to ventilate the patient or extubate at the end of the procedure.
d. Obstructive sleep apnea has a close association with neurologic disorders, especially after acute events. The disorder is often underdiagnosed or patients are not using their prescribed devices. Close observation in monitored units is usually warranted after anesthesia.
e. Renal insufficiency. Neurosurgical patients are often exposed to large volume challenges that can either overload or severely dehydrate them. Therefore, baseline renal function plays an important role.
(1) Contrast media are an important cause of renal deterioration. Risk factors include pre-existing renal disease, diabetes, heart failure, presence of hypotension or pressors, old age, anemia, and volume of contrast used. Proven preventing methods include generous hydration and use of low-osmolality contrast media. There is evidence for administration of steroids, N-acetylcysteine, and sodium bicarbonate but efficacy is still to be determined. Mannitol and furosemide should be avoided [12].
f. Patients with diabetes mellitus are to be instructed to continue all long-acting insulins and stop the oral agents the morning of surgery. Close monitoring is warranted throughout the perioperative period.
g. Vascular diseases. Presence of femoral, aortic or carotid disease or grafts has obvious implications for the interventional radiologist as well as for the anesthesiologist in obtaining adequate venous or arterial access.
h. History of seizures and associated medications. Anticonvulsants interact with cytochrome enzymatic systems. They can induce (fosphenytoin), have no effect (levtiracetam), or inhibit (valproate) them. In general, enzyme-inducing medications will show resistance to neuromuscular blockers and opioids. Preoperative adherence to regimens should be reinforced and drug levels sometimes are warranted.
i. Coagulation disorders. Baseline coagulation tests are usually ordered in all cases, despite problematic evidence, usually because patients will require anticoagulation and because any amount of abnormal bleeding in the tight space of the brain can be disastrous.
j. Medication reconciliation
(1) Day-of-surgery status of β-blockers, antiseizure medications, and antibiotics.
(2) Narcotic or drug history indicates a patient tolerant to opioids. Increased doses of medication are necessary in order to obtain the same clinical effects. Conversions to different opioid medications, nonopioid adjuvants, and nerve blocks can be helpful. Caution is warranted since these patients do not exhibit the same degree of tolerance to the adverse respiratory effects of opioids.
k. Nothing per mouth status is relative and might be hard to elicit, especially in cases of emergencies, for patients with decreased awareness, immobile in bed for various periods of time, or actively vomiting. We recommend a conservative approach geared toward an expeditious protection of the airway.
l. Allergy history. Especially important are:
(1) History of heparin-induced thrombocytopenia (thrombosis and thrombocytopenia in relation with heparin administration) should be elicited. Confirmatory tests usually performed in association are antigen tests (ELISA) and functional assays (serotonin). Alternatives to heparin like direct thrombin inhibitors (hirudins) exist but they are harder to dose, monitor, and reverse.
(2) Iodinated agent allergy (shellfish, internal vs. external). Considering that a true allergy to iodine is technically impossible (vital body constituent) and most common shellfish allergies are in fact directed against a protein, it is at least as important to enquire about other food allergies or asthma [13].
(3) Protamine, derived from salmon, has also been linked with a higher incidence of hypersensitivity reactions, especially in patients with histories of fish allergy, exposure to neutral protamine hagedorn insulin or vasectomy.
3. Any anesthesiologic interaction should include a physical examination with emphasis on:
a. The possibility of difficult airway. Depending on the neurologic status, sometimes the only information that can be obtained is about the external appearance of the face (retrognathic mandible), neck (thickness, length), and dentition (missing or loose teeth, presence of prosthesis).
b. Rapid, focused neurologic examination. We can encounter a wide range of presentations, from quasinormal ambulatory patient to the unconscious, critically ill after a possibly fatal hemorrhage. In the former, the primary provider has already performed a thorough neurologic examination, which is present in the medical chart and should be reviewed. The anesthesiologist’s examination should document significant findings (signs and symptoms of increased intracranial pressure or the presence of lateralization syndromes) and alert of any interval changes. In the latter, unconscious patient, we should review the size of pupils and response to light, response to noxious stimuli, presence of abnormal reflexes, and if possible rough motor and sensory examination.
c. While the primary provider is very likely focused on the neurologic aspects of the patient, we should not forget about the rest of the body. Cardiac examination (murmurs and arrhythmias, neck bruits), pulmonary examination (wheezes and rales), presence of scars, to cite just a few, are key findings.
C. Intraoperative considerations
1. Monitoring
a. All patients undergoing anesthesia care should be monitored according to the standards of basic anesthesia monitoring of the American Society of Anesthesiologists. While for patients undergoing general anesthesia, compliance is generally very good, every effort should be undertaken to extend similar care to the patients scheduled for monitored anesthesia care.
b. Cardiovascular monitors
(1) In addition to the standard monitors, intracranial procedures necessitate an invasive arterial line in order to facilitate
(a) beat-to-beat blood pressure monitoring during rapid intraoperative changing conditions of high stimulation (induction of anesthesia, head pinning, incision, sudden blood loss, emergence) interspersed with lower-intensity periods (angiography, lesion exposure, closure);
(b) frequent arterial blood gases and point-of-care hemoglobin and electrolyte checks;
(c) the necessity to place an arterial line before induction is usually directly proportional to general physical status of the patient.
(2) Central venous catheters are encouraged for hemodynamic monitoring and administration of medications especially for larger AVMs. When performed, ultrasound guidance, maximal sterile conditions, and Seldinger technique with pressure transduction are recommended.
c. Precordial Doppler is used for craniotomies in sitting position (for AVMs of the posterior fossa) to monitor for a venous air embolism. See Chapter 26 for details on venous air embolism.
d. Cerebral and neurophysiologic monitors
(1) Bispectral index monitoring or full-standard electroencephalogram is sometimes employed in relation to total intravenous anesthesia or institution of burst suppression.
(2) Jugular venous oxymetry is an invasive measure of jugular bulb blood saturation, intermittent or continuous with oxymetric catheters. Generally physicians are concerned with low values, lower than 55% that signal insufficient oxygen delivery. In the case of AVMs, due to the presence of shunt, the values are actually excessively high, 80% to 90%. One can monitor in real time the success of embolization or resection by observing the decrease in the abnormally high values. It has also been used to monitor the limit of safely induced hypotension (see chapter 28).
(3) Transcranial Doppler demonstrates higher flow velocity and decreased pulsatility in the feeding arteries of medium and large AVMs. The method has diagnostic and monitoring value but is less sensitive for small lesions. Post treatment, whether surgical or embolization, the flows are reliably decreased and pulsatility index increases, resembling normal arteries. Intraoperative uses are usually harder to implement due to logistical reasons (see chapter 28).
(4) Awake testing and neurophysiologic monitoring (somatosensory, motor, auditory-evoked potentials, or electromyographic recordings). In cases of AVMs located in language, motor, or sensory areas, precise dissection will offer a better chance at preserving function. In awake patients, intracarotid (Wada test) or superselective injection of amobarbital (inhibits grey matter), lidocaine (inhibits white matter), and even propofol (5 to 10 mg doses) permits testing of any deficits that may appear. For patients under general anesthesia, one has to employ various neurophysiologic monitors to obtain the same results. Before resection or embolization, electrical stimulation or provocative medication is administered and the neural pathways are monitored for significant changes. The anesthesiologist has to be vigilant because occasionally intraoperative seizing can be triggered. Treatment is propofol 1 mg/kg and flooding the area with sterile cold saline [14,15] (see chapter 26).
(5) Cerebral oxymetry values can be skewed by the presence of AVMs. After careful baseline calibrations, it can be used to detect vascular complications related to catheter manipulation during neuroendovascular procedures or for monitoring of cerebral oxygenation during induced hypotension.
7
2. Anesthetic regimen [16]. The typical modern neuroanesthetic regimen uses volatile or intravenous agents plus a narcotic. By and large, for the average patient, both regimens are similar in regards to the main characteristics of an ideal neuroanesthetic: amnesia, cardiovascular stability, rapid emergence, good operating conditions. More important is the practitioner’s familiarity with a specific one. There might be subtle differences in regards to a specific patient with a specific abnormality but definitive studies are lacking. Some of their pros and cons are listed below:
a. Inhalation agents’ advantages include ease of titration, long track record, lower cost, and fast emergence. The main disadvantage is the direct cerebral vasodilation that offsets the decrease in flow associated with decreased cerebral metabolism.
b. Intravenous anesthetic are beneficial because they provide cerebral vasoconstriction, less postoperative nausea and vomiting, and less interference with neurophysiologic monitoring. They have more unpredictable pharmacokinetics so there is potential for longer emergence due to overdosage and the opposite of intraoperative awareness due to underdosage. Also, there can be severe acidosis from the propofol-infusion syndrome and shift of structures during stereotactic surgery due to decreased cerebral blood volume.
c. In one of the very few studies that compared patients with AVMs, isoflurane versus propofol-based anesthesia groups did not differ in terms of awakening times and early recovery of motor and respiratory functions. In the same note, both groups had impairment of higher cognitive functions for up to 24 hours after anesthesia [17].
d. In analyzing a subgroup of patients that had total intravenous anesthesia for their craniotomies, those with intravascular disorders (including AVMs) still had the highest rates of early postoperative complications, for an aggregate of 76.5% when adding postoperative shivering, hypertension, nausea, and vomiting [18].
CLINICAL PEARL
For AVMs, hypertension on induction is less likely to cause rupture and hemorrhage than for an aneurysm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree