Women by a 3:2 ratio are more likely to have a SAH than men [3].
B. Outcome. Approximately 15% of patients with acute SAH do not survive to hospital admission. Of those who do, 25% will die within the first 24 hours, 40% by day 7, and 50% by 1 year. Only 20% of those who survive to hospital discharge will be able to return to their previous lifestyle and 25% will have significant cognitive deficits that require assistance to perform some daily tasks [1,4,5]. Forty percent of these patients will have major neurologic deficits that require continuing medical care in a long-term care facility [1]. Prognostic factors for an unfavorable outcome are increasing age, worsening neurologic grade (Tables 9.2 and 9.3), ruptured posterior circulation aneurysm, increasing aneurysm size, increased hemorrhage size and systolic hypertension on admission. Medical conditions associated with a less favorable outcome include hypertension, myocardial infarction, liver disease, or SAH [6,7].
C. Patient characteristics
1. Risk factors. Risk factors for SAH are determined by genetics, lifestyle, and aneurysm size.
a. Genetic factors. Genetic factors influence the location of the aneurysm. However, the strongest determinant of risk is familial. The relative risk of having a cerebral aneurysm is 2.15 (CI 1.77 to 2.59) when one first-degree relative and 51.0 (8.56 to 1,117) when two first-degree relatives have an aneurysm [8]. This association has led to public health organization providing voluntary screening of all first-degree relatives of patients who have had a SAH. Other genetic diseases such as polycystic kidney disease, Ehlers–Danlos type IV, Moyamoya disease, all heritable connective tissue disorders, and any heritable coagulopathies are associated with an increased risk of cerebral aneurysm [2].
b. Lifestyle. Lifestyle choices, smoking, use of cocaine or amphetamines and frequent, significant alcohol use are all associated with increased risk of SAH. In patients under the age of 30 years, the most common cause of SAH is cocaine and amphetamine use although there have been reports of excessive use of cold medicine containing phenylephrine or derivatives leading to hypertension and eventual SAH. The most common associated medical condition is poorly controlled or uncontrolled hypertension. This constitutes a very large risk group, since according to the Centers for Disease Control (CDC) approximately 31% of the population over 20 years has hypertension.
c. Risk of rupture. In a patient with an aneurysm, the risk of rupture depends on the size and location. Aneurysms in the anterior circulation make up 97% of most aneurysms and they are distributed in the following manner (approximate values): Ophthalmic (1%), posterior communicating (25%), internal carotid (5%), anterior communicating artery (ACOA [41%]), A2 segment of the anterior cerebral artery (2%), and middle cerebral artery (24%). The posterior circulation has only 3% to 4% of all aneurysms; they occur on the basilar (30%) and posterior inferior cerebellar artery (70%) [9]. The location and size of the aneurysm is important for anesthesia management because it will determine the patient’s position and the risk of rupture during intraoperative or neurointerventional treatment. The risk of rebleeding is approximately proportional to the aneurysm size and location and is similar to the risk of spontaneous rupture [9,10].
II. Medical presentation
A. Presenting symptoms. The classic description of a SAH is the patient’s statement “the worst headache of my life.” This statement may or may not be accompanied by focal neurologic symptoms. Symptoms that typically accompany SAH are [2,11]:
1. nausea and vomiting
2. meningismus
3. decreased level of consciousness
4. focal neurologic signs
Atypical presentations include seizure, confusion, and a fall which can confuse the diagnosis since it suggests head trauma rather than SAH. In about 20% of patients the only symptom is an atypical or mild prodromal headache and half of these patients (10%) will be misdiagnosed as migraine or tension headache. Patients with a missed diagnosis have much poorer overall outcome as they usually have a major rebleed shortly after the prodromal event. All patients with a “thunderclap headache” or other unusual neurologic complaints need an immediate CT scan. Patients with a prodromal headache that is misdiagnosed are a major concern for the anesthesiologist. When this patient population has subsequent SAH, they are likely to have vasospasm at the time of diagnosis. BP management of possible vasospasm and an unsecured leaking aneurysm requires precise control [2,12,13].
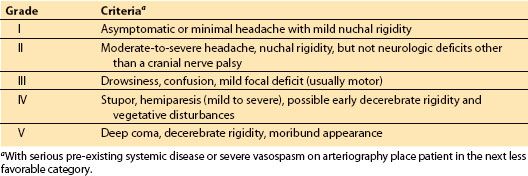
B. Severity of hemorrhage. SAH grading scales provide accurate prognostic information to assist in decision making by the patients, their family, and the physicians providing care. Hunt and Hess Classification of Status (Table 9.1) and the World Federation of Neurosurgeons (WFNS) SAH scale (Table 9.2) are the current standard assessment tools. It is not possible to assign the ASA physical status classification without an understanding of these evaluation tools. The Hunt and Hess (H&H) grade is unique in that it incorporates the pre-existing medical conditions into the scoring. Patients with serious systemic disease (e.g., hypertension, diabetes, chronic pulmonary disease, cardiac disease) or with vasospasm on angiogram will be moved to the next less favorable score (e.g., from II to III). The WFNS SAH scale utilizes the Glasgow coma scale (GCS) to assess neurologic status. Although often under recognized by anesthesiologist, the Fisher Grade for SAH (Table 9.3) classifies the amount of blood seen on computerized axial tomography (CT) scan of the brain. It is very useful in assessing the risk of vasospasm and delayed ischemic neurologic deficit (DIND) [11,14,15]. This knowledge is very important for the immediate medical and anesthesic management.
Table 9.1 Hunt and Hess grade of patient status after SAH
1
III. Associated physiologic complications. Many severe and minor medical complications are associated with the severity of the SAH. While much is made of the morbidity and mortality caused by vasospasm and delayed cerebral ischemia (DCI), the associated medical complications are the causative factors in mortality and morbidity. The conditions that have the highest association with severe morbidity and mortality occur as an immediate and direct consequence of SAH and are present often on admission. Many problems are correctable (e.g., hyperglycemia, fever) and although evidence is lacking that correction of these issues will unequivocally prevent poor outcome, there is strong evidence that it will improve outcome [16]. The proportional contributions to mortality and significant morbidity are medical complications (23%), vasospasm and DCI (23%), rebleeding (22%), multiple factors (34%) [15]. Knowledge and appropriate management of medical complications associated with SAH by the anesthesiologist will contribute to better outcomes and make planning and management decisions more effective. The extent and consequences of the medical complications are found in Figure 9.1. High-risk, high-frequency physiologic perturbations that have recognized impact on anesthesia management are discussed. Anesthetic management will extend past the initial securing of the aneurysm either in IR or OR as follow up procedures for treatment for vasospasm and high ICP (ventriculoperitoneal shunt, decompressive craniectomy) [17] and on rare occasions clipping of additional aneurysms [5,11].
CLINICAL PEARL
Intraventional radiology (IR) procedures: Fluid overload and renal injury due to the volume of dye administered can be managed by monitoring urine output and administering N-acetylcysteine and NaHCO3.
A. Cerebral. Cerebral autoregulation, the ability to maintain a stable cerebral perfusion or cerebral blood flow (CBF) relatively constant over a range of BP, is dependent on the size of the hemorrhage, the ICP, and the time since the SAH. The basic principles of autoregulation are discussed elsewhere (Chapter 1). Immediately after SAH, impairment is directly related to the degree of injury determined by the H&H grade [5]. Immediately after rupture, patients with an H&H grade of I and II would be expected to have normal autoregulation in areas of their brain that are not directly affected by the SAH. In patients with an H&H grade of III or more, the lower limit where autoregulation fails (hypotension) is significantly higher. Failures of autoregulation during relative hypo- or hypertension increase cerebral blood volume, ICP, and impair perfusion causing cerebral ischemia. The vascular response to changing CO2 and hypoxia remains normal immediately after injury when H&H grade is less than III. Cerebrovascular reactivity impairment is seen in all patients with vasospasm and this impairment begins prior to development of vasospasm. An entirely normal response to changing PaCO2 or PaO2 is unlikely to be present >24 hours after rupture [18]. The principles regarding elevation in ICP due to the sudden increase in intracranial volume are the same as covered in Chapter 27.
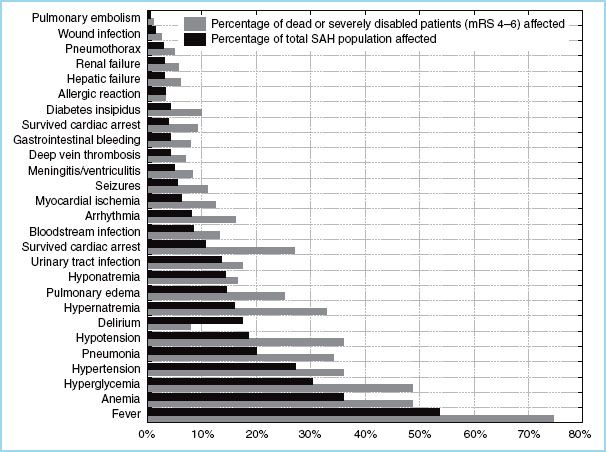
FIGURE 9.1 A comparison of the complication profiles of 576 patients with the subgroup of patients (220). Source: Wartenberg KE, Schmidt JM, Claassen J, et al. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med. 2006;34:617–623.
1
B. Cardiac. Cardiac abnormalities are the most commonly seen associated injury and occur immediately after SAH. They fall into two categories: Those that produce cardiac arrhythmias and those that decrease myocardial contractility. The etiology of these effects is believed to be the sudden catecholamine surge that accompanies the SAH. Catecholamines produce marked vasoconstriction and an accompanying increase in systemic and pulmonary BP. Autopsy findings in previously healthy patients describe diffuse microinfarctions distributed throughout the myocardium. This effect exacerbates underlying cardiac disease seen in the fifties and older age groups [11,14,19]. Some cardiac findings predict poor-quality outcomes.
Table 9.2 World federation of neurosurgeons (WFNS) SAH scale
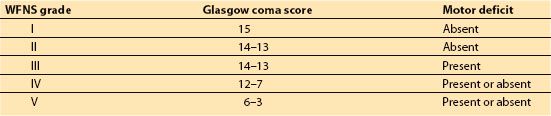
Table 9.3 Fisher grades for computed tomography (CT) findings in SAH

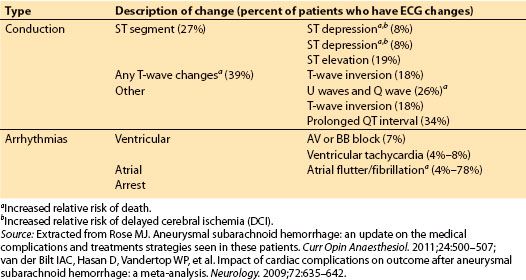
1. Arrhythmias. Between 60% and 100% of patients will have ECG conduction abnormalities after SAH. Most are not indicators of eminent cardiac catastrophe and will resolve spontaneously within 3 days. Arrhythmias linked to ischemic myocardial injury are associated with increased relative risk of death and DCI. The most commonly seen ECG changes are listed in Table 9.4.
Considering the percent of patients who experience cardiac arrhythmias, they represent a fairly benign finding that can serve as an alert to examine the patient carefully for other signs of cardiac injury [11,14,19].
Table 9.4 Compilation of common cardiac rhythm and conduction abnormalities seen in SAH
2. Myocardial injury. Catecholamine release is believed to cause the decreased myocardial contractility producing decreased cardiac output [20]. Elevations in troponins (35% to 83%) and brain nauretic protein (BNP) (45% to 92%) are frequently seen and are indicators of considerable increased relative risk of death and morbidity and predict vasospasm and DCI [11,16,19]. Transthoracic echo is used to detect and monitor the severity of the myocardial injury. This may not be possible during the emergency period immediately after the SAH. Thus, identifying the clinical syndrome of neurogenic stress cardiomyopathy or “stunned myocardium” is important for anesthesia management. It presents with chest pain, dyspnea, hypoxia, and often cardiogenic shock and appears within hours of the SAH. It usually lasts up to 3 days. It is believed to be the cause of sudden death in up to 12% of patients. Medical management is supportive; the most important components are maintaining euvolemia, catecholamine support when needed, and BP control. Monitoring of cardiac output is recommended using transthoracic (TTE) or transesophageal echocardiography (TEE) when available and appropriate [16]. The indicators of increased relative risk of death and DCI are [19].
a. Elevated BNP—Death: 11.1 (CI 4.7 to 26.0), DCI: 4.5 (CI 1.8 to 11.4)
b. Elevated troponins—Death: 2.0 (CI 1.2 to 3.4), DCI: 3.2 (CI 2.3 to 4.4)
c. Wall motion abnormalities—Death: 1.9 (CI 1.2 to 2.9), DCI: 2.1 (CI 1.2 to 3.8)
d. Selected rhythm abnormalities (see Table 9.4)
3. Blood volume. Patients experience a diuresis and are hypovolemic prior to administration of loop and osmotic diuretics [16]. Hypovolemia contributes to the secondary hypoperfusion brain injury. The Neurocritical Care Society Multidisciplinary Consensus Conference recommended goals should be euvolemia, not hypervolemia as was previously recommended. Hypervolemia even when used after securing the aneurysm is associated with significant patient harm. Fluid replacement with an isotonic crystalloid has moderate consensus support [16]. Anesthetic management goals should be consistent with these recommendations.
CLINICAL PEARL
Hypovolemia: Most patients are hypovolemic prior to the medically induced diuresis, consequently maintaining intravascular volume with normal to hyperosmotic solutions like balanced salt or normal saline solutions, 5% albumin or in some organizations, hetastarch is appropriate.
1
C. Pulmonary. Pulmonary symptoms occur in about 20% of patients after SAH but there is evidence of impaired oxygenation in about 80% [16]. Immediately postSAH, the most likely cause is neurogenic or cardiogenic pulmonary edema while later in the patient’s course aspiration pneumonitis, acute respiratory distress syndrome, and transfusion-related acute lung injury (TRALI) becomes more likely [21]. Impaired oxygenation may manifest as lower than expected SpO2. The proposed mechanism is direct lung injury due the catecholamine storm at the time of SAH or acute right-sided failure from myocardial injury. Immediately postSAH, apneic oxygen reserve is likely to be significantly decreased and the oxygen reserve decreases as the H&H grade increases [16]. About 14% of patients with SAH will have pulmonary edema [14]. This lack of oxygen reserve must be anticipated when planning the most appropriate method to secure the airway.
1
D. Glucose metabolism. Abnormal glucose metabolism, hypo- and hyperglycemia are predictive of outcome with hyperglycemia being one of three independent medical management predictors of mortality and morbidity [14]. Patients with admission serum glucose values below 160 mg/dL had a 33% mortality whereas glucose values above 160 mg/dL and above 230 mg/dL increased mortality to 71% and 95%, respectively [22]. Hyperglycemia has also been identified as an independent predictor of symptomatic vasospasm, permanent neurologic disability, and death within 3 months [23–25]. Most studies suggested that glucose value of greater than 140 mg/dL is associated with increased neurologic complications. Glucose control also decreases nonneurologic medical problems, for example, infection, pneumonia, and sepsis [15]. Identifying the ideal glucose values is complex since some research studies utilizing microdialysis catheters in patients after SAH found tissue glucose was significantly lower than serum glucose values [26]. Recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference suggests maintaining serum glucose between 80 mg/dL and 200 mg/dL and carefully avoiding hypoglycemia, considered serum glucose <70 mg/dL [16,26]. American Diabetes Association recommends use of an insulin infusion for maintenance of serum glucose between 140 mg/dL and 180 mg/dL in critically ill patients [27].
2
E. Electrolytes. Electrolyte abnormalities are common postSAH. They are exacerbated by therapeutic interventions like osmotic diuresis with mannitol, loop diuretic, and contrast dye.
1
1. Sodium. Hyponatremia, serum Na <130 mEq/L, occurs in about 30% of patients after a SAH and is the most common electrolyte imbalance in patients with SAH. It is associated with an inappropriate loss of Na triggered by the release of either natriuretic peptide (cerebral salt wasting syndrome [CSWS]) or antidiuretic hormone (syndrome of inappropriate antidiuretic hormone [SIADH]). CSWS causes a hypovolemic hyponatremia by increasing renal Na loss in excess of water. It is treated by carefully administering hypertonic saline; usually 0.9% NaCl is adequate to replace volume and Na losses although hypertonic saline can be used. Volume replacement is a critical factor in therapy for CSWS. SIADH causes a euvolemic hyponatremia from water retention in excess of Na loss. It is treated by administration of small doses of a loop diuretic with fluid replacement to maintain euvolemia. Hyponatremia has not been associated with poor outcomes [11,13,15]. Hypernatremia, serum Na >150 mEq/L, is an iatrogenic disease caused by the use of osmotic and renal loop diuretics. It has not been associated with poor outcomes [16].
2. Calcium, magnesium, potassium. Hypocalcemia occurs in about 41% to 74% of all patients after SAH and is assumed to be associated with the diuresis caused by the SAH, administration of contrast and diuretics. It is not associated with documented neurologic complication but may contribute to hypotension when combined with calcium-channel blockers. Although not frequently measured, low serum Mg concentrations are assumed to accompany hypocalcemia. Many organizations have elected to preemptively treat vasospasm or DCI with MgSO4 infusion making hypermagnesiumemia more likely. Mg counteracts Ca effects and can further decrease cardiac contractility, BP, and potentiate nondepolarizing muscle relaxants. Hypokalemia is usually a complication of diuretic therapy. The usual considerations of arrhythmogenesis apply.
F. Pituitary hormones. Hypopituitarism occurs in 37% to 55% of patients who survive the initial illness. Deficiencies of growth hormone and adrenocorticotropic hormone are the most commonly reported. Patient quality of life rather than outcome is affected by these deficiencies. Anesthesiologists are most likely to encounter this problem when this population returns for shunt revisions or elective ablation of additional aneurysms. Primary operative concerns would be inadequate cortisol, inadequate steroid response to surgical stress, and increased possibility of hypoglycemia. Both can be managed with the preemptive use of steroids. Diabetes insipidus, deficiency of antidiuretic hormone, presents prior to initial hospital discharge and is relatively rare [28,29].
IV. Vasospasm and delayed cerebral ischemia. Cerebral vasospasm and DCI occur in 70% of patients who survive the initial hemorrhage and 20% to 40% of this group becomes symptomatic. Symptoms usually begin by 72 hours after the initial hemorrhage. Early in the recovery period, vasospasm and the ischemic consequences remain the most common cause of death and severe disability.
A. Diagnosis. While the gold standard for the diagnosis of vasospasm is an arteriogram, most clinicians use a combination of transcranial doppler (TCD) and neurologic signs as the trigger for initiating an arteriogram [30]. TCD uses ultrasound to evaluate arterial blood flow in the anterior and posterior circulation. Multiple daily examinations evaluate the vasculature for an increase in arterial flow velocity. Increasing velocity is diagnostic of vasospasm. Common neurologic symptoms include altered level of consciousness and new focal neurologic finding, although recognized risk factors play a role. Some organizations perform a “screening” arteriogram based on risk alone [5,16,18]. Using the Fischer scale, the odds ratio (OR) for developing systematic vasospasm when compared to Fischer grade 0 to 1 patients is grade 2 and 3— OR 1.6, and grade 4—2.2 OR [18]. The decision to proceed to IR for assessment and treatment is organization dependent [16] and varies from routine time intervals to only with severe neurologic deterioration (see Chapter 28).
B. Preemptive treatment. Causation of vasospasm and DCI is not entirely clear. Accepted knowledge suggests the hemoglobin molecule disrupts the balance between endothelin, a vasoconstrictor, and nitric oxide, a vasodilator, but this does not explain the wide variation in severity, response to therapy, and outcome. Research into genetic mechanisms may produce major changes in management [32,33]. Preemptive treatment of vasospasm and DCI is a priority and may need to be initiated by the anesthesia team in the OR. It should be continued during all subsequent anesthetics until therapy has been discontinued. Meta-analysis strongly supports that preemptive therapy decreases the incidence of vasospasm and DCI but treatment does not change outcomes [31].
1. Calcium-channel blockers. Nimodipine is the only drug with a clearly demonstrated benefit in reducing the incidence and severity of vasospasm. Nicardipine, an intravenous substitute, does not have the same demonstrable benefits [25,32]. Nonetheless, most patients will receive one of these drugs. Nicardipine is an effective antihypertensive and is very useful in controlling hypertension immediately post SAH and before securing the aneurysm. Nicardipine can lead to significant hypotension during anesthesia induction and maintenance. There are no clear guidelines defining whether to continue nicardipine during general anesthesia. Since hypotension should be studiously avoided, management is up to the individual practitioner with the consensus goal to continue nicardipine whenever possible.
CLINICAL PEARL
Vasospasm: Preemptive treatment and therapeutic control of vasospasm is based on intravenous infusion of nicardipine between 2.5 mg/h and 10 mg/h. Hypertension, hypervolemia, and hemodilution (triple H or HHH) therapy has been replaced by normovolemia, maintaining hemoglobin between 10 g/dL and 11 g/dL and selective use of hypertension in patients with neurologic changes due to vasospasm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







