Upper GI bleeding
Peptic ulcer disease
Gastritis
Esophageal/gastric varices
Mallory-Weiss tear
Boerhaave’s syndrome
Malignancy
Lower GI bleeding
Diverticulosis
Angiodysplasia
Malignancy
Inflammatory bowel disease
Ischemic colitis
Infectious colitis
Hemorrhoids
Anal fissure
Solitary rectal ulcer
Upper Gastrointestinal Bleeding
Case Vignette
A 78-year-old female is admitted to the intensive care unit after a motor vehicle collision. She is diagnosed with a subdural hematoma and is intubated due to a low Glasgow Coma Score (GCS). On hospital day 3 she acutely develops bright red, bloody output from her nasogastric tube that is associated with hypotension and tachycardia.
Upper GI bleeding is characterized as bleeding proximal to the ligament of Treitz including the stomach, duodenum, and esophagus. Patients with upper GI bleeding may present with symptoms such as hematemesis (vomiting of fresh, bright red blood, coffee ground emesis) or melena (passage of black or tarry stools). It may also be occult and present with hemoccult positive stools or as a microcytic anemia. Symptoms of upper GI bleeding may include light-headedness, orthostatic hypotension, and syncope related to blood loss and hypovolemia. Upper GI bleeding is estimated to be five times more common than lower GI bleeding [16]. Mortality from upper GI bleeding has also been shown to increase with age [3, 15].
The most common causes of upper GI bleeding in the elderly are peptic ulcer disease and gastropathy accounting for between 55–80 % of cases [17]. Esophagitis and esophageal/gastric varices account for the majority of the remaining cases of upper GI bleeding in the elderly population. Other causes of upper GI bleeding include esophageal tears due to Boerhaave’s syndrome or Mallory-Weiss syndrome, duodenal diverticula, Dieulafoy’s ulcers, angiodysplasia, hemobilia, aortoenteric fistulae, and neoplasms. History should focus on prior episodes of peptic ulcer disease, diagnosis or treatment for Helicobacter pylori, smoking, alcohol use, previous abdominal surgeries, and steroid use. Liver disease is another key risk factor and may suggest variceal bleeding. Critically ill patients are also prone to upper GI bleeding due to stress gastritis, with patients receiving mechanical ventilation, and those admitted with traumatic brain injury, severe burn, or trauma at significantly increased risk. Medication history including anticoagulants, antiplatelet agents, and recent use of nonsteroidal anti-inflammatory drugs (NSAIDs) should be obtained.
Lower Gastrointestinal Bleeding
Case Vignette
An 84-year-old male on Plavix presents after a syncopal fall. He is found to have sinus tachycardia during your initial evaluation. On exam, he is noted to have guaiac positive stool.
Lower GI bleeding is defined as bleeding from a location distal to the ligament of Treitz. While lower GI bleeding is less common than upper GI bleeding, there remains a high incidence of lower GI hemorrhage among the elderly. It is important to remember that one of the most common sources of blood per rectum is from the upper GI tract, and therefore, upper GI bleeding should always be considered and ruled out. Obtaining a detailed history of bleeding events is crucial and should include the color and quantity of the blood passed per the rectum. Patients should be queried regarding any previous history of colon malignancy, diverticulosis, and personal or family history of inflammatory bowel disease. Nearly 80 % of patients presenting with lower GI hemorrhage will stop bleeding without intervention; however, the recurrence rate can be as high as 25 % [18]. Similar to upper GI bleeding, mortality in patients with lower GI bleeding has been shown to increase with age [19].
The most common cause of lower GI bleeding is diverticulosis of the colon which is usually characterized by abrupt onset and usually painless hematochezia. Lower GI bleeding is also frequently caused by colonic neoplasms where ulceration of the mucosal surface by the tumor results in bleeding. Bleeding from colonic neoplasms is often more subtle than diverticular bleeding and may lead to slow blood loss over a prolonged period of time. Colitis from infection or ischemia may also lead to lower GI bleeding. Patients with inflammatory bowel disease also frequently experience lower GI bleeding which is associated with bloody diarrhea and crampy abdominal pain. Lastly, angiodysplasia is a common cause of lower GI bleeding, especially in the elderly, accounting for up to 30 % of lower GI bleeding in patients over the age of 65 [20]. Anorectal bleeding should also be considered and may be characterized by bright red bleeding from hemorrhoids, solitary rectal ulcer, or anal fissures.
Obscure Gastrointestinal Bleeding
GI bleeding from a source that is not identified by either colonoscopy or esophagogastroduodenoscopy (EGD) is referred to as obscure GI bleeding. In these cases bleeding may be from a small bowel source or may originate from a source in the upper GI tract or colon that was not visualized during prior diagnostic attempts. While obscure GI bleeding is less common than a defined upper or lower GI source, this cause of bleeding is often challenging to diagnose and treat and may lead to ongoing blood loss with prolonged hospital stays. The most common sources of obscure GI bleeding among the elderly are angioectasias and tumors [21, 22].
Diagnosis
Endoscopy is the diagnostic and therapeutic intervention of choice for intestinal hemorrhage.
Angiography is a good alternative to endoscopy for both diagnostic and therapeutic intervention in patients with relatively brisk GI bleeding and is a good, less invasive alternative to surgery in older patients.
Nuclear scintigraphy, CT enterography, and capsule endoscopy are preferred diagnostic modalities for obscure and slow GI bleeding.
Endoscopy
GI endoscopy, including EGD and colonoscopy, is the diagnostic, as well as therapeutic, procedure of choice for diagnosing GI bleeding. EGD is safe and effective in the elderly population and has been shown to deliver important diagnostic information in over 90 % of patients [4]. Colonoscopy is also effective with similar rates of completion and higher diagnostic yield compared to younger patients. However there is a higher rate of exams limited by poor preparation and a higher rate of complications and perforations in patients ≥80 years [21, 23]. Because the elderly are less likely to tolerate large volume preps and are at higher risk of serious adverse effects such as life-threatening electrolyte abnormalities, severe dehydration, and acute kidney injury, the American Society for Gastrointestinal Endoscopy (ASGE) recommends against the use of sodium phosphate and magnesium-based preps and for the use of a split-dosage balanced polyethylene glycol-based preparation solution [21]. Colonoscopy and EGD generally require conscious sedation to ensure patient comfort and to allow for the technical completion of the study. Although elderly patients tolerate unsedated EGD better than their younger counterparts particularly if using a small caliber scope, sedation is generally well tolerated in the elderly patient and significantly increases test completion [21, 24]. The elderly can be more sensitive to the effects of periprocedural medications, and studies have demonstrated a significantly higher incidence of desaturations in geriatric patients during sedation for endoscopy when compared to younger patients [21, 25]. Because of this the ASGE recommends lower initial doses and slower titration of procedural medications for endoscopy in elderly patients [21]. Elderly patients may also be at increased risk of aspiration during invasive procedures and tolerate such events poorly due to underlying lack of physiologic reserve.
Angiography
Angiography is another useful modality in the diagnosis of GI bleeding. Angiography allows for localization of the bleeding source when the rate of bleeding is as low as 0.5 ml/min. Diagnostic angiography is available in most medical centers and can be performed via arterial puncture and catheter placement, usually via the femoral artery. Diagnostic angiography is safe and well tolerated with risks including puncture site complications including bleeding, hematoma, and pseudoaneurysm formation. There is also risk of acute kidney injury associated with the administration of iodinated intravenous contrast [26]. In addition to its diagnostic capabilities, angiography also possesses the added benefit of potential therapeutic intervention. Catheter-based interventions including vasopressin injection and angioembolization will be discussed later in this chapter.
Nuclear Medicine
Nuclear scintigraphy can be used to identify the source of GI bleeding and involves Tc-99 m-labeled red blood cells or technetium-99 m (Tc-99 m) sulfur colloid. Radiolabeled red blood cells maintain their activity for longer periods of time compared to injection of Tc-99 m sulfur colloid, allowing for serial imaging, and may give better results when used to diagnose lower GI bleeding. Nuclear scintigraphy is safe, noninvasive, and an accurate method of identifying GI bleeding from any source in the GI tract [27]. The benefits of this imaging technique for detecting GI bleeding include its sensitivity for very slow bleeding, with the ability to detect bleeding rates as low as 0.1 ml/min. Additionally, it is noninvasive [28].
Computed Tomographic Scanning
Contrast-enhanced multidetector-row helical computed tomography (MDCT) scanning is a newer technology which can be used to identify GI bleeding by using the images obtained during the arterial phase to identify extravasation of contrast into the GI tract (Fig. 17.1). Areas concerning on the arterial phase can be further investigated using delayed images to assess for residual contrast, or pooling of contrast, which further supports the presence of active arterial bleeding. Like angiography MDCT appears to be most effective in detecting active or symptomatic GI bleeding; however unlike angiography, MDCT has been reported to detect bleeding rates as low as 0.1 ml/min, similar to that for nuclear scintigraphy [6]. It also has the advantage of being noninvasive, is widely available, avoids the risks associated with arterial puncture required for angiography, and has the added benefit of identifying extraluminal pathology or causes of hemorrhage. However MDCT is associated with risks of IV contrast administration and radiation exposure [27]. Using MDCT to diagnose the source of bleeding may be an efficient method to facilitate directed interventions via either endoscopy or angiography and may improve the ability to localize bleeding and increase the diagnostic and therapeutic yield of angiography [29, 30].
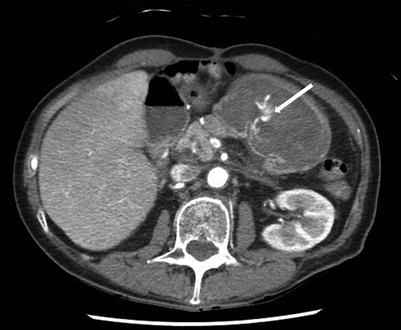

Fig. 17.1
Upper GI bleeding diagnosed by contrast-enhanced computed tomographic (CT) scan. The arrow indicates an area of active arterial extravasation seen in the stomach
CT Enterography
As the technology of CT scanning improves, the sensitivity of imaging with the advent of multidetector imaging systems with 64 channels has improved image resolution and introduced more applications for the use of CT scans in the diagnosis of GI bleeding. CT enterography utilizes orally delivered, neutral contrast material that improves the visualization of mucosal pathology. In most CT enterography protocols, oral contrast is given in several doses in 20 min intervals in order to distend the lumen of the intestine. With the neutral background provided by the enterally delivered contrast material, active hemorrhage can be visualized and localized more easily than traditional CT scanning protocols. The delivery of the volume of oral contrast required to perform CT enterography does place elderly patients at risk of aspiration. CT enteroclysis is another option which delivers contrast enterally at a continuous rate via a nasojejunal tube which is placed under fluoroscopic guidance, potentially decreasing the risk of vomiting, reflux, and aspiration [31].
Magnetic Resonance Imaging
The use of magnetic resonance imaging (MRI) in the diagnosis of GI bleeding continues to evolve; however, it is not currently a mainstay of diagnosis for bleeding localization. The use of MRI for localization of bleeding in occult GI bleeding has shown a diagnostic yield of 40 % with improved localization of pathology in the distal small bowel compared to the proximal small bowel [32]. MRI enterography has low diagnostic yield for identifying intraluminal small bowel bleeding sources causing occult GI bleed; however, this imaging modality has been shown to identify extraintestinal pathology which may aid in the diagnosis of the cause of hemorrhage [33]. Further studies are needed to define the role of MRI in the diagnosis of occult GI bleeding; however, it may be a useful imaging adjunct to consider in patient with bleeding sources not localized by other, more traditional imaging techniques.
Capsule Endoscopy
Improvements in technology have allowed for the more widespread use of capsule endoscopy for the diagnosis of GI bleeding. The capsule, which is the size of a large pill, is swallowed and travels the entire length of the GI tract through peristalsis. Capsule endoscopy is especially useful in identifying obscure sources of GI bleeding and can allow visualization of the small intestine with imaging completed to the cecum in nearly 75 % of cases [34]. Capsule endoscopy is a purely diagnostic test with a yield near 50 % but does not allow for intervention [35–37]. Clinical studies evaluating the effectiveness of capsule endoscopy for obscure GI bleeding have shown a diagnostic yield between 30 and 69 %; however diagnostic yield increases with increasing age and is highest in patients ≥85 years [22, 38–40]. Most likely the higher diagnostic yield is due to a higher incidence of pathology in elderly patients, with the most common lesion found being angioectasia [21, 22]. The test is well tolerated and safe, with retained capsule representing the most significant risk associated with this procedure [41, 42].
Management
Management of the elderly patient with GI bleeding should include appropriate invasive and noninvasive hemodynamic monitoring and fluid resuscitation.
Aspiration precautions should be enacted and consideration given to early and/or prophylactic intubation.
Careful medication history should be taken with attention paid to antiplatelet and anticoagulant medications.
Assessment of coagulopathy should be performed, and any abnormalities corrected with platelet and plasma transfusions, adjuncts such as prothrombin complex concentrates, and tranexamic acid can be considered.
Endoscopy is the preferred diagnostic and treatment modality for intestinal hemorrhage in the elderly, with excellent efficacy and safety.
Complications, in particular perforation during colonoscopy, are more common in elderly patients and should be kept in mind during procedures and during post-procedural monitoring.
In patients where endoscopy has failed to control hemorrhage, angiography and surgery can be considered. Angiography is less invasive and associated with lower rates of complications and so may be more attractive for frail elderly patients; however it is also associated with higher rates of rebleeding.
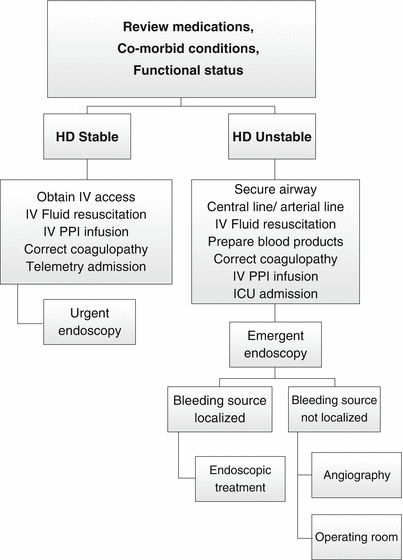
Fig. 17.2
Algorithm for the management of upper GI bleeding
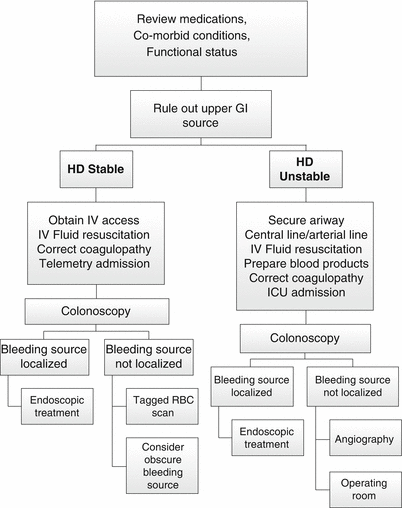
Fig. 17.3
Algorithm for the management of lower GI bleeding
Initial Evaluation
As with any acute illness associated with blood loss, the initial assessment of GI bleeding should include evaluation of the airway, breathing, and circulation. The patient should be evaluated in the appropriate level of care with frequent monitoring of vital signs. Patients should have two large bore IVs placed for transfusion of blood or IV fluids as needed. The primary treatment goals in patients with suspected GI bleeding include adequate resuscitation, diagnosis of the cause of GI bleeding, localization of bleeding, and treatment of the bleeding source with endoscopy, angiography, or surgery. A nasogastric tube may be placed and saline lavage performed to give some insight as to whether the bleeding is originating from an upper GI source. A Foley catheter should be placed to monitor urine output and assess the response to fluid resuscitation. If the index of suspicion is high for an upper GI source of bleeding, high-dose proton pump inhibitor infusion therapy should be initiated.
Aspiration Risk in Elderly
Elderly patients who are hospitalized with GI bleeding have been shown to experience complications early in their hospital course, frequently within 96 h of admission [43]. Among the most frequent complications in the elderly are pneumonia and aspiration [17]. Therefore, airway protection and pulmonary toilet are of significant importance during the resuscitation and hospitalization of patients with GI bleeding.
All patients should be administered supplemental oxygen and the head of bed should remain elevated at all times. Supplemental oxygen by nasal cannula is an important adjunct at the time of endoscopy as it has been shown to prevent hypoxemia, oxygen desaturation, and cardiac arrhythmias [44]. Consideration should be given to early, prophylactic intubation in order to secure the airway and limit the risk of aspiration. This is especially true in patients undergoing endoscopy who will be receiving conscious sedation. Elderly patients generally require lower doses of benzodiazepine sedative medications than their younger counterparts [21, 45]. Elderly patients may experience unexpected respiratory arrest that may necessitate emergent endotracheal intubation further increasing the risk of aspiration. Elderly patients may also experience paradoxic reactions to conscious sedation which may result in altered mental status making endoscopic interventions difficult [15]. Current guidelines from the ASGE recommend heightened attention to the dose and effects of standard sedatives used during endoscopy on the elderly and emphasize the importance of lower initial doses of sedatives with more gradual titration [21].
Fluid Resuscitation
Normal saline and lactated Ringer’s are the most common resuscitation fluids used in the treatment of hypovolemic shock. Studies comparing the use of normal saline and lactated Ringer’s in patients with acute hemorrhage show equivalent outcomes [23, 46]. There is a theoretical risk of hyperkalemia with the use of lactated Ringer’s which may be exacerbated in patients with acute kidney injury or chronic renal insufficiency seen in many elderly patients. Resuscitation with colloids has theoretical benefits over crystalloid resuscitation due to its ability to restore intravascular volume more efficiently due to the higher oncotic pressure resulting in decreased losses into the extravascular space. This may be beneficial in elderly patients as colloid resuscitation may allow for lower total infusion volume required to restore perfusion pressure. Unfortunately several studies have been performed to compare crystalloid versus colloid resuscitation showing no clear statistical benefit in patients receiving colloid resuscitation [47, 48]. A large randomized controlled trial compared 3497 patients who received 4 % albumin to 3500 patients receiving normal saline [24]. This study found no significant difference in mortality, need for renal replacement therapy, or hospital length of stay suggesting no benefit in the use of colloid resuscitation compared to crystalloid resuscitation.
Recent research from the trauma population suggests that early transfusion of blood products may be beneficial in patients with acute hemorrhage. Not only does early transfusion of packed red blood cells (PRBCs) appear to be superior to crystalloid resuscitation in patients with hemorrhage, but the transfusion of other blood components may be beneficial as well. Studies suggest that transfusion of fresh frozen plasma (FFP) and platelets in addition to PRBCs may decrease mortality [18]. The optimal transfusion strategy appears to be a 1:1 ratio of FFP to PRBCs [27]. Studies also suggest that increasing the ratio of platelets to PRBCs may be beneficial as well [49].
Medications
Elderly patients who are diagnosed with GI bleeding should be queried regarding their current medication regimen with particular focus on antiplatelet and anticoagulant medications. Laboratory studies including complete blood count, PT/INR, and PTT should be measured to diagnose any derangements in the clotting cascade. Classical measures of coagulopathy may not address all coagulation abnormalities and thus may fail to correctly diagnose coagulopathy. The classical measures fail to measure platelet dysfunction due to medications or fibrinolysis. Limitations of these traditional laboratory measures of coagulopathy have led to increased interest in the use of alternative measures of coagulation, clot strength, and fibrinolysis. Thromboelastography (TEG) and thromboelastometry (ROTEM) are an established method for measuring the viscoelastic properties of blood for hemostasis testing [12, 20, 32]. TEG/ROTEM has the benefit of providing detailed information on clot formation and clot strength and provides results more rapidly than conventional measures of coagulation.
Treatment with fresh frozen plasma (FFP) is a common method used to reverse anticoagulation in patients taking Coumadin. FFP is effective; however it takes time to transfuse and may require the infusion of large volumes of fluid in order to correct the INR. In elderly patients who are actively bleeding, strategies need to be considered that more rapidly correct the coagulopathy and prevent large volume infusions that may be problematic in patients with comorbid disease such as congestive heart failure. Pharmacologic agents such as Factor VIIa and prothrombin complex concentrates (PCC) should be considered as they result in rapid correction of the clotting abnormality and require minimal volume infusion.
Recombinant Factor VIIa has traditionally been used as a treatment for uncontrolled bleeding in patients with hemophilia. It has been used to treat GI bleeding in patients with liver disease and in the treatment of trauma-induced coagulopathy [31, 33]. Randomized control trials have failed to show improvements in mortality in cirrhotic patients with acute upper GI bleeding compared with placebo [50, 51]. There are case reports which suggest that recombinant Factor VIIa may be a useful strategy in elderly patients with GI bleeding who are poor candidates for aggressive intervention; however, there are concerns regarding increased thromboembolic complications, particularly those affecting the arterial circulation; further randomized controlled trials are needed to further define its role in treating acute GI bleeding in the elderly [52]. Several studies have compared the use of PCC to FFP and vitamin K for reversal of coagulopathy following injury or in anticipation invasive procedures. These studies have concluded that PCC corrected the INR more quickly and effectively than the combination of FFP and vitamin K. Because of these studies, several guidelines advocating the use of PCCs to reverse coagulopathy in cases of acute bleeding have been published. A study of patients with life-threatening hemorrhage, including GI bleeding (32 %), studied adherence to these guidelines and found that adherence to guidelines for PCC reversal (PCC ≥20 IU/kg +vitamin K dose ≥5 mg within 8 hrs) resulted in a two-fold decrease in 7-day mortality [53]. A further study comparing PCC to FFP for reversal of warfarin in patients with GI bleeding found a greater reduction in INR, reduced active bleeding, and a decrease in the need for invasive procedures in the PCC group [54]. Further studies are needed assess safety and to better define the role of PCC in the treatment of elderly patients with GI bleeding, but it should be part of the armamentarium in the treatment of bleeding patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





