Fig. 35.1
Posterior view of an anatomic model depicting the insertion site for an interlaminar epidural injection at the L5-S1 level
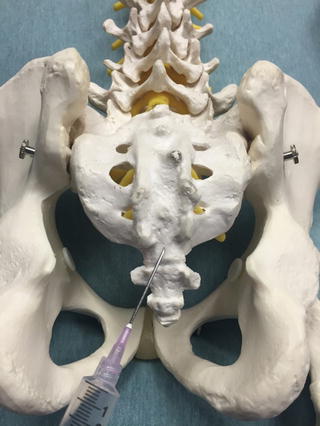
Fig. 35.2
Posterior view of an anatomic model depicting the insertion site for a caudal epidural injection
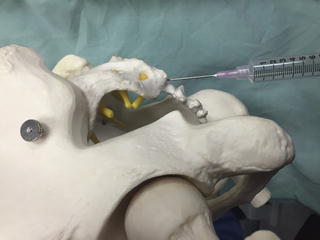
Fig. 35.3
Lateral view of an anatomic model depicting the insertion site for a caudal epidural injection
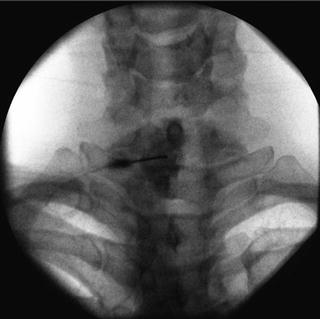
Fig. 35.4
AP fluoroscopic image, post-contrast, of an interlaminar epidural injection at the C7-T1 level
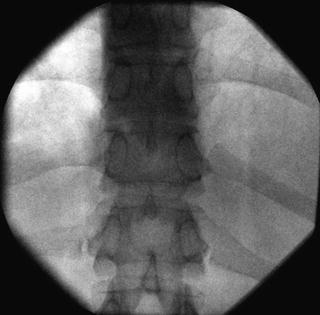
Fig. 35.5
AP fluoroscopic image of the thoracic vertebrae
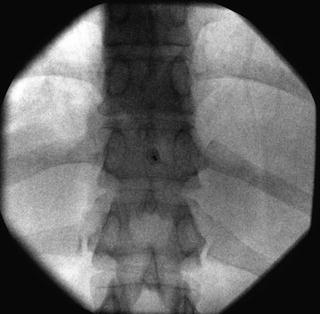
Fig. 35.6
AP fluoroscopic image of the thoracic vertebrae depicting proper interlaminar needle placement at the T10-T11 level
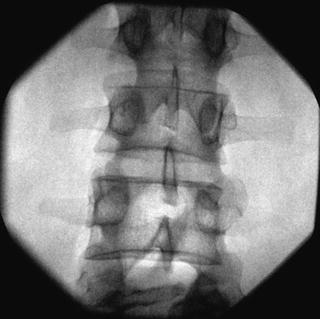
Fig. 35.7
AP fluoroscopic image of the lumbar vertebrae
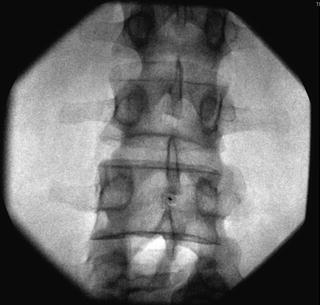
Fig. 35.8
AP fluoroscopic image of the lumbar vertebrae depicting proper interlaminar needle placement at the L3-L4 level
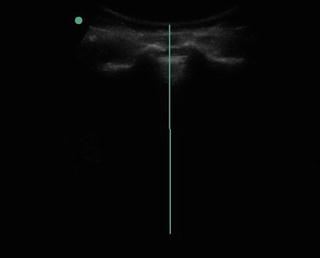
Fig. 35.9
Ultrasound image of the Sacral Cornua and the Sacral Hiatus for placement of caudal epidural steroid injection
Epidural steroid injections introduce glucocorticoids, typically mixed with an anesthetic agent, via spinal needle into the epidural space (a potential space that lies between the ligamentum flavum and the dura) to relieve pain. Generally, it relieves radicular symptoms more than axial symptoms. Pain relief can also be attributed in part to the “wash out” effect, whereby the volume of injected material disperses the inflammatory molecules as well as the anesthetic agent that the steroid is mixed with [8].
Most guidelines recommend utilization of image guidance, typically in the form of fluoroscopy, with use of a non-iodinated contrast agent administered prior to injection of glucocorticoid, to ensure proper needle placement as well as lack of vascular uptake [5]. Image guidance is utilized in conjunction with the “loss of resistance” technique in interlaminar injections. The loss of resistance method can be performed with a glass or plastic syringe, using either air or saline, or with no syringe at all (hanging drop technique). Guidelines recommend cervical interlaminar injections be performed at the C7-T1 level and no higher than the C6-C7 level, as the cervical epidural space is widest at the C6-T1 levels and gaps in the ligamentum flavum become more common in the ascending cervical levels [9].
Caudal epidural injections can be guided with either fluoroscopy or ultrasound. A caudal epidural injection is considered to be the least specific modality of the three epidural options, requiring high volumes of medication to reach the pathological area in the spine [10]. What caudal epidural injections lack in specificity, it makes up for in ease and safety, as these procedures can typically be performed in the outpatient clinic setting under ultrasound guidance with minimal risk of dural puncture [1]. Trained specialists, many with fellowship training in pain and/or interventional spinal procedures, typically perform epidural injections including but not limited to anesthesiologists, physiatrists, and interventional radiologists. Indications for epidural glucocorticoid injections include: acute radiculopathy, subacute/chronic radiculopathy, spinal stenosis, and post-spine surgery syndrome [1].
Multiple glucocorticoid agents have conventionally been utilized for epidural injections, which include dexamethasone, hydrocortisone, methylprednisolone, triamcinolone, and betamethasone. For many interventionalists, the steroid preference is based on personal preference as well as spinal level of the procedure. Triamcinolone and betamethasone have particles that can form aggregates, which can occlude a blood vessel if inadvertent intravascular uptake occurs [11]. Interventionists may prefer dexamethasone, which is non-particulate and thereby does not aggregate, depending on the spinal level injected and/or proximity to the vasculature.
Pathophysiology
Anatomy
Spina l nerves roots exit posterolaterally through the neural foramina, above the vertebral level in the cervical spine and below the vertebral level in the thoracic and lumbar spine. Neural impingement can occur from spinal canal and foraminal stenosis, from spondylosis and spondylolisthesis, as well as from disk herniations and other structures compressing the nerve root along the path of exit.
Radiculopathies
Greater than 95% of lumbar disk herniations occur at the L4-L5 and L5-S1 levels, followed by the L3-L4 and L2-L3 levels. Consequently, the L5 and S1 nerve roots are most commonly affected [12]. Additionally, posterolateral disk herniations of the nucleus pulposus are the most common form of disk herniation, as this is the weakest area of the annulus fibrosus. This can result in nerve root irritation proximal to the neural foramen, as it descends in the lateral recess [12]. In contrast, lateral or extra-foraminal herniations affect the nerve root after it exits the neural foramen which can result in nerve root irritation at the same disk level. For example, a far lateral L4-L5 disk herniation would cause L4 neural impingement, not L5 as in posterolateral herniation.
In addition to disk herniation causing foraminal narrowing, spondylosis can also decrease the diameter of the foramen, which results in similar symptomatology. Lastly, central disk herniations, as well as spondylosis and congenital canal stenosis can affect any portion of the spinal canal [12]. It is thought that a localized inflammatory reaction or mechanical compression leads to radicular symptoms. This allows for a potential intervention to halt the inflammatory cascade in acute, subacute, and chronic radiculopathies.
Spinal Stenosis
Much like a radiculopathy, spinal stenosis pain can result from mechanical compression and/or local inflammation of the nerve root. With spinal stenosis, however, nerve root ischemia can also occur, which results from venous congestion and arterial insufficiency. This can lead to symptoms of neurogenic claudication (i.e., leg numbness, heaviness, tingling, pain, and/or weakness with walking and/or standing) [12].
Post-Laminectomy Syndrome (Failed Back Surgery Syndrome )
This chronic pain syndrome, that persists despite surgical intervention predominantly effecting the lumbar spine, has multiple names (e.g., failed back syndrome, post-laminectomy syndrome) and has a constellation of etiologies that result in continued low back and/or leg pain. Typically, the surgery that precedes this syndrome is a spinal fusion or laminectomy and the differential diagnosis of the resulting pain can be grouped based on whether the predominance of pain is in the back or in the leg(s) [13].
Clinical Considerations
Much like any pain complaint, back/neck pain should be evaluated with a thorough history and physical examination, which can often lead to the diagnosis before any additional imaging studies are utilized. “Red Flags” with respect to spinal pain require prompt management to reduce morbidity and mortality. These include, but are not limited to, history of trauma or cancer, suspected fracture, unintentional weight loss, progressive leg weakness, urinary/bowel incontinence, unremitting pain, suspected myelopathy, suspected cauda equina syndrome, and saddle anesthesia [12]. The presence of such signs and symptoms may also indicate the need for a surgical referral.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





