INFECTIOUS DISEASE EMERGENCIES
NICOLAUS W.S. GLOMB, MD, MPH AND ANDREA T. CRUZ, MD, MPH
GOALS OF EMERGENCY CARE
Fever is one of the most common presenting complaints for children seen in the emergency department (ED). ED physicians face the challenge of differentiating potentially life-, limb-, or sensory-threatening causes of infection from the vast majority of children with febrile illnesses that will spontaneously resolve without intervention. For infectious disease (ID) emergencies, the clinical evaluation should focus upon prompt recognition of potentially serious conditions. Evidence-based diagnostic strategies can facilitate care and avoid unnecessary evaluations of otherwise well-appearing children.
RELATED CHAPTERS
Signs and Symptoms
• Septic Appearing Infant: Chapter 68
Clinical Pathways
• Fever in Infants: Chapter 87
• Fever in Children: Chapter 88
• Pneumonia, Community-Acquired: Chapter 90
Medical, Surgical, and Trauma Emergencies
• Cardiac Emergencies: Chapter 94
• Gastrointestinal Emergencies: Chapter 99
• Gynecology Emergencies: Chapter 100
BACTEREMIA AND SEPSIS
CLINICAL PEARLS AND PITFALLS
• It can be difficult to differentiate among children with uncomplicated viral infections and occult bacteremia.
• With reduction in vaccine-preventable diseases, most positive blood cultures are false positive with contaminants.
• Evidence-based guidelines can optimize management in the young febrile child.
Current Evidence
The epidemiology of pediatric bacteremia has changed dramatically over the last three decades due to widespread use of the pneumococcal conjugate and Haemophilus influenzae type B (Hib) vaccines. The most common isolates now causing bacteremia are listed in Table 102.1. While rates of pneumococcal bacteremia have declined in the postpneumococcal conjugate vaccine era, Streptococcus pneumoniae still comprises most cases of bacteremia, along with Staphylococcus aureus, Salmonella, group A streptococcus (GAS), and meningococcus; group B streptococcus (GBS) and Gram-negative rods remain the most common causes of bacteremia and sepsis in neonates and young infants. In some studies, contaminants are up to sevenfold more common than true pathogens. As a consequence, use of evidence-based algorithms in the approach to the febrile child (Chapters 87 Fever in Infants and 88 Fever in Children) can reduce unnecessary evaluation and optimize treatment of the non–toxic-appearing child.
Goals of Treatment
The goals of treatment are to recognize which children may be at higher risk for bacteremia than the general pediatric population (e.g., asplenic children, children with central venous catheters [CVC], neutropenic children) and to be cognizant of the most common organisms causing bacteremia seen in a given region. Thus, knowledge of local antibiotic resistance patterns is critical for the ED physician.
Clinical Considerations
Clinical recognition: Children at highest risk for bacteremia are under 2 years of age. For meningococcus, biphasic peaks occur: one during infancy and a second during adolescence. Thus, algorithms for fever management focus heavily upon young children due to higher incidence at this age and because the signs of occult bacteremia are difficult to discern. In many tertiary care centers, the children at highest risk for bacteremia and sepsis are children with indwelling CVC, neutropenia, or short gut. These children may have baseline tachycardia from anemia, making triage recognition more problematic.
Triage considerations: Recognition of abnormal vital signs (e.g., tachycardia, hypothermia) and signs of poor perfusion are critical for rapid initiation of resuscitation in the ED. Given the variation in normal vital sign ranges through the pediatric age spectrum, recognition can be facilitated if alerts are built into electronic health records.
Clinical assessment: Several studies have attempted to stratify the risk of bacteremia and other serious bacterial infections in febrile children. Table 102.2 describes clinical and laboratory predictors of occult bacteremia in young children.
TABLE 102.1
MOST COMMON CAUSES OF PEDIATRIC OCCULT BACTEREMIA IN THE POSTPNEUMOCOCCAL CONJUGATE VACCINE ERA
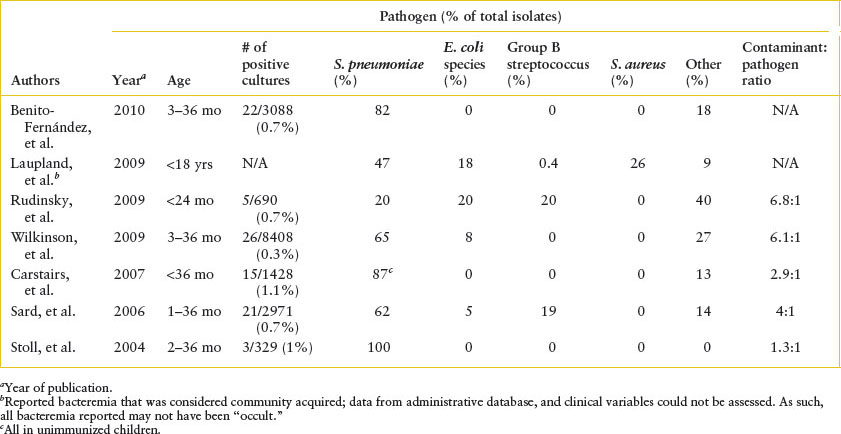
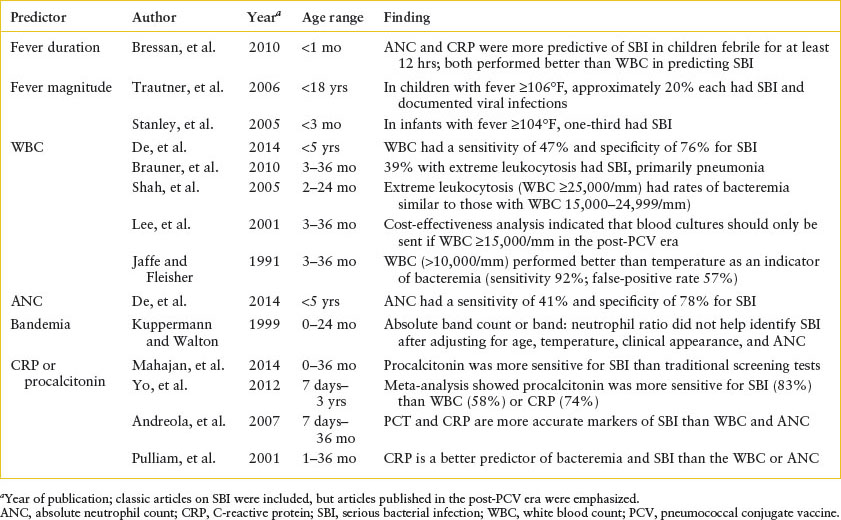
TABLE 102.2
CLINICAL AND LABORATORY PREDICTORS OF SERIOUS BACTERIAL INFECTION
Management: Empiric antibiotic management depends upon knowledge of the most common causes of bacteremia in a community. While there has been a decline in pneumococcal isolates causing occult bacteremia, there have been increases in the rates of penicillin- and cephalosporin-resistant pneumococcal isolates. As such, if there is concern for invasive pneumococcal disease, initiating treatment with vancomycin and a third-generation cephalosporin (cefotaxime or ceftriaxone) would be appropriate. In the event that the isolate is cephalosporin susceptible, a cephalosporin would be a much more effective bactericidal drug than vancomycin. However, if resistance to a cephalosporin is present, then the child is receiving a drug to which the isolate retains susceptibility. This regimen would also provide coverage for the most common other causes of bacteremia and sepsis in immunocompetent children outside the neonatal period. If staphylococcal disease is suspected, children could receive nafcillin as well; in the event that they have methicillin-susceptible S. aureus (MSSA), nafcillin is far more bactericidal than vancomycin. In immunocompromised hosts, the causes of bacteremia are more diverse, and antipseudomonal coverage should be considered a priori. In addition, these children may also be at higher risk for antibiotic-resistant organisms from prior antibiotic exposure(s). Reviewing prior culture data to evaluate for a history of infection with drug-resistant organisms can help optimize ED-based management. Empiric coverage with vancomycin and an antipseudomonal beta-lactam (e.g., ceftazidime, piperacillin/tazobactam, or ticarcillin/clavulanate) may be considered.
OTHER SYSTEMIC INFECTIOUS EMERGENCIES
Goals of Treatment
The goals of treatment are to rapidly identify children at risk for some less common pathogens that can result in fulminant infection and to plan an empiric treatment course while awaiting diagnostic testing.
CLINICAL PEARLS AND PITFALLS
• If rickettsial disease is suspected based upon epidemiologic risk factors and clinical presentation, doxycycline should be started immediately.
• Doxycycline is the treatment of choice for the most deadly rickettsial disease in the United States, Rocky Mountain spotted fever (RMSF); this is true for all age groups.
• The “classic” triad for RMSF of fever, headache, and a rash is present in approximately 60% of cases.
• Babesiosis presents with symptoms similar to malaria, but in a child who lacks a travel history to a malaria-endemic region.
• Asplenic patients are at highest risk for babesiosis complications.
Toxic Shock Syndrome
Toxic shock syndrome (TSS) is characterized by severe, prolonged shock and is caused by toxins produced by S. aureus or GAS. TSS presents with the sudden onset of high fever, vomiting, and watery diarrhea. Pharyngitis, headache, and myalgias may also occur, and oliguria rapidly develops. Within 48 hours, the disease progresses to hypotensive shock. The patient has a fever, usually 39° to 41°C (102.2° to 105.8°F); a diffuse, erythematous maculopapular rash; and hyperemia of the mucous membranes. In almost one-half of cases of streptococcal TSS, no portal of entry is identified, or only minor, nonpenetrating skin trauma is identified in retrospect. The Centers for Disease Control and Prevention (CDC) definitions of TSS are described in  e-Table 102.1. Laboratory findings include leukocytosis with a left shift, thrombocytopenia, transaminitis, elevated creatinine, elevated creatinine kinase, myoglobinuria, and coagulopathy. Complications can include acute respiratory distress syndrome (seen in over one-half of patients), acute kidney injury occurs in almost all children (creatinine elevation precedes hypotension), and disseminated intravascular coagulation. The initial diagnosis is clinical. The following laboratory tests should be obtained from all children suspected of having TSS: CBC, platelet count, PT, PTT, d-dimer, electrolytes, blood urea nitrogen (BUN), creatinine, AST, alanine aminotransferase (ALT), and creatinine kinase. Cultures of the blood, urine, stool, throat, and vagina serve to isolate S. aureus and to rule out other infectious causes of shock. A lumbar puncture (LP) is often required to exclude bacterial meningitis. The management of TSS is the same as that for shock caused by other organisms (see Chapter 91 Shock). Broad-spectrum antibiotics (vancomycin and ceftriaxone) are indicated for patients who are hemodynamically unstable, while those who are less ill may have treatment limited to an antistaphylococcal agent. Many authorities recommend the addition of clindamycin, which inhibits the toxin.
e-Table 102.1. Laboratory findings include leukocytosis with a left shift, thrombocytopenia, transaminitis, elevated creatinine, elevated creatinine kinase, myoglobinuria, and coagulopathy. Complications can include acute respiratory distress syndrome (seen in over one-half of patients), acute kidney injury occurs in almost all children (creatinine elevation precedes hypotension), and disseminated intravascular coagulation. The initial diagnosis is clinical. The following laboratory tests should be obtained from all children suspected of having TSS: CBC, platelet count, PT, PTT, d-dimer, electrolytes, blood urea nitrogen (BUN), creatinine, AST, alanine aminotransferase (ALT), and creatinine kinase. Cultures of the blood, urine, stool, throat, and vagina serve to isolate S. aureus and to rule out other infectious causes of shock. A lumbar puncture (LP) is often required to exclude bacterial meningitis. The management of TSS is the same as that for shock caused by other organisms (see Chapter 91 Shock). Broad-spectrum antibiotics (vancomycin and ceftriaxone) are indicated for patients who are hemodynamically unstable, while those who are less ill may have treatment limited to an antistaphylococcal agent. Many authorities recommend the addition of clindamycin, which inhibits the toxin.
Rickettsial Diseases
The most severe endemic rickettsial disease in the United States is RMSF, caused by Rickettsia rickettsii. Transmitted by dog and wood ticks, RMSF is found in the southeastern United States and most cases present during the spring and summer months. Fever, headache, and a rash are considered the characteristic triad of RMSF, but are found in only 60% of cases. The rash begins as a maculopapular rash on the wrist and ankles and progresses centrally, later becoming petechial. Laboratory findings include thrombocytopenia, hyponatremia, and transaminitis. Multisystem involvement is seen with this systemic vasculitic condition, and the high mortality rate (up to 80% in untreated patients) usually is attributable to disseminated intravascular coagulation and shock. As a consequence, treatment with doxycycline should begin immediately if RMSF is suspected, without awaiting confirmatory diagnostics (acute and convalescent serologies). Doxycycline use has been shown to decrease morbidity and mortality over the second-line drug, chloramphenicol, and doxycycline also treats ehrlichiosis, which can present with symptoms similar to RMSF. Doxycycline is the preferred treatment for RMSF in children of all ages, unless a child has a severe doxycycline allergy.
Babesiosis
Babesiosis is caused by Babesia microti, an intraerythrocytic parasite whose symptoms mimic those of malaria in persons who lack a travel history to a malarial-endemic region. Babesiosis is seen in the northeastern and upper Midwestern United States; it is transmitted by the same Ixodes ticks that transmit Lyme disease and has also been transmitted via blood transfusion. Symptoms include fever and influenza-like illness; signs can be minimal, but in more severe cases, tachypnea, hypotension, icterus, and mild hepatosplenomegaly can be seen. Disease can be severe in asplenic patients, who have very high parasite burdens. The diagnosis is made by thick and thin blood smears demonstrating the organism’s classic Maltese cross form within erythrocytes. Treatment is clindamycin and quinine for 7 to 10 days. Exchange transfusion may be needed for patients with parasitemia above 10%.
CNS INFECTIOUS EMERGENCIES
Meningitis, Bacterial
CLINICAL PEARLS AND PITFALLS
• The most common causes of meningitis in the first month of life are GBS and Gram-negative rods; beyond the first month of life, the most common etiologies are pneumococcus and meningococcus.
• The “classic” signs and symptoms of meningitis, including nuchal rigidity, are insensitive in infancy.
• The Gram stain of the cerebrospinal fluid (CSF) should be used to broaden, but not to narrow, empiric antibiotic selection.
• Empiric antibiotic therapy should comprise bactericidal agents that cross the blood–brain barrier. For neonates, ampicillin and either cefotaxime or ceftriaxone can be used. For infants and older children, vancomycin (for enhanced pneumococcal coverage) and either cefotaxime or ceftriaxone (for meningococcal coverage) should be utilized.
• In neonates and young infants with a CSF pleocytosis, addition of acyclovir (20 mg/kg every 8 hours) is reasonable until herpes simplex virus (HSV) is excluded.
Current Evidence
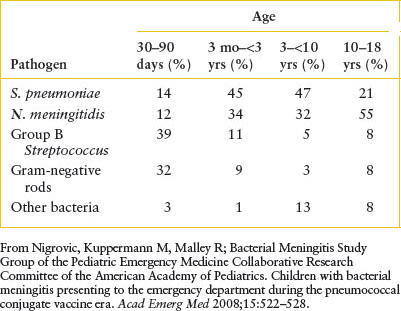
The most common causes of bacterial meningitis by age are listed in Table 102.3. In the first month of life, Escherichia coli and GBS are usually isolated; Listeria monocytogenes, a Gram-positive rod, accounts for 1% to 3% of the cases. Between 30 and 60 days of age, GBS continues to be recovered frequently, followed by S. pneumoniae and Neisseria meningitidis; Hib occurs rarely. After the first 2 months of life, S. pneumoniae and N. meningitidis cause the majority of meningeal infections; Haemophilus influenzae remains a consideration primarily among children not immunized with conjugated Hib vaccine. Salmonella, an uncommon etiologic agent in the United States, should be suspected in the first few months of life if meningitis occurs in association with gastroenteritis. The incidence of acute bacterial meningitis has declined in the last three decades due to widespread use of the Hib and polyvalent pneumococcal conjugate vaccines.
Goals of Treatment
The goal of treatment is the rapid recognition and treatment of bacterial meningitis to decrease a child’s risk of neurologic sequelae. The clinical team should consider neuroimaging prior to LP in the immunocompromised child or the child with focal neurologic deficits. Clinical outcomes include time to appropriate parenteral antibiotics, CSF sterility at 24 to 48 hours, and neurologic outcome.
TABLE 102.3
ETIOLOGIES OF ACUTE BACTERIAL MENINGITIS CHILDREN OUTSIDE THE NEONATAL PERIOD
Clinical Considerations
Clinical recognition: The most common signs and symptoms of bacterial meningitis are listed in Table 102.4. Before 2 to 3 months of age, the history is usually that of irritability, an altered sleep pattern, vomiting, and decreased oral intake. In particular, paradoxical irritability points to the diagnosis of meningitis. Irritability in the infant without inflammation of the meninges is generally alleviated by maternal fondling; however, in the child with meningitis, any handling, even directed toward soothing the infant, may increase irritability by its effect on the inflamed meninges. The amount of time spent sleeping may either increase because of obtundation or decrease from irritability. Bulging of the fontanelle, an almost certain sign of meningitis in the febrile, ill-appearing infant, is a late finding. Vomiting is a sensitive but nonspecific feature of infantile meningitis.
TABLE 102.4
SIGNS AND SYMPTOMS OF MENINGITIS
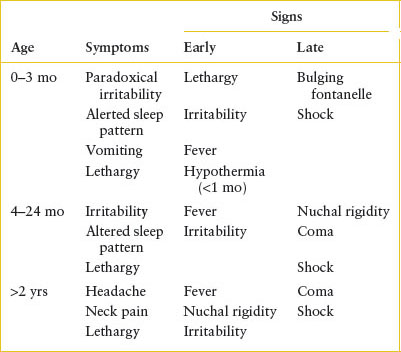
As the child ages past 3 months, the symptoms gradually become more specific for involvement of the central nervous system (CNS). A change in the level of activity is almost always noticeable. However, it is only in the child older than 2 years that meningitis manifests reliably with complaints of headache, neck stiffness, and photophobia.
The physical examination in the young infant rarely provides specific corroboration, even when the history suggests meningitis. Fever may be absent in these children, despite the presence of bacterial infection. Any child younger than 2 to 3 months who is brought to the ED with a documented temperature of ≥100.4°F should be considered at risk for meningitis. The physical signs are sufficiently elusive that many experts caution that one should not rely exclusively on the examination to rule out meningeal infection. In several studies, 5% to 10% of these young infants had meningitis (although mostly aseptic), despite being judged clinically well by experienced clinicians.
After 2 to 3 months of age, increasing, but not absolute, reliance can be placed on the physical findings; fever is typically noted. Specific evidence of meningeal irritation is often present, including nuchal rigidity and, less often, Kernig (pain with extension of the leg on a flexed femur) and Brudzinski (involuntary lifting of the legs when the head is raised while the child is lying supine) signs. When an LP fails to confirm the diagnosis of meningitis, despite the presence of meningeal signs, other conditions must be pursued that can mimic the findings on physical examination. Conditions capable of producing the findings typical of meningismus (irritation of the meninges without pleocytosis in the CSF) include severe pharyngitis, retropharyngeal abscess (RTA), cervical adenitis, arthritis or osteomyelitis of the cervical spine, upper lobe pneumonia, subarachnoid hemorrhage, pyelonephritis, and tetanus.
Seizures are a presenting complaint for 20% of children with bacterial meningitis. Many of these are focal, recurrent, or prolonged seizures. Most clinicians advise that children younger than 6 months with a first-time febrile seizure should routinely have LP performed to discern the presence of CNS infection, unless there are specific contraindications or an alternative diagnosis is readily apparent. Febrile seizures are reviewed in Chapters 26 Fever and 67 Seizures.
Triage considerations: Children with fever and altered mental status or neck pain should be evaluated promptly for meningitis. Associated tachycardia and hypotension can be seen in children with meningitis who are in compensated or uncompensated shock, respectively. If meningococcus is suspected, providers should wear simple face masks and utilize droplet precautions.
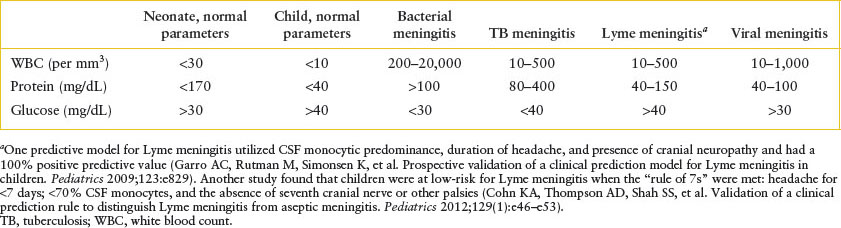
Clinical assessment: Initial considerations in the management of children with bacterial meningitis are listed in Table 102.5. Confirmation of meningitis is by sampling of the CSF. The most common CSF parameters associated with various causes of meningitis are summarized in Table 102.6. The CSF Gram stain will be positive for an organism in approximately two-thirds (40% to 90%) of cases of bacterial meningitis and the results of Gram stain should be used to add additional antimicrobial therapy when appropriate. It is generally prudent to await culture confirmation before antibiotic coverage is narrowed. In certain patients, computed tomography (CT) should be considered prior to LP. These criteria are not as well defined for pediatric patients, but in adult patients, they include immunocompromised state; history of focal CNS disease; presence of papilledema; and focal neurologic deficit.
TABLE 102.5
IMMEDIATE MANAGEMENT STEPS FOR CHILDREN WITH SUSPECTED OR CONFIRMED BACTERIAL MENINGITIS
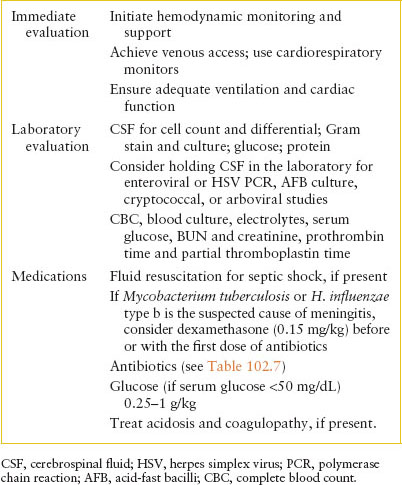
Seizures occur in 20% of children with bacterial meningitis and, occasionally, in those with viral infections of the CNS, such as meningoencephalitis due to HSV. One should always be suspicious of derangement of the glucose or sodium as a cause of convulsive activity. However, most seizures are caused by irritation of the brain from the infectious process. They are controlled using routine antiepileptic agents. Subdural effusion and, less often, empyema occur in 20% to 40% of young children with meningitis but usually appear later in the course.
Management: The optimal antibiotics for empiric treatment of acute bacterial meningitis would offer coverage for the most common pathogens, be bactericidal, and cross the blood–brain barrier. Treatment options for meningitis in normal hosts are described in Table 102.7. Treatment of tuberculosis meningitis and HSV meningitis is described elsewhere. Evaluation and treatment of meningitis in immunocompromised hosts (e.g., human immunodeficiency virus [HIV]-infected children) should be undertaken with consultation with an ID specialist. Consideration should be given to admitting children with suspected bacterial meningitis to an intensive care unit setting for close initial monitoring. Standard precautions are indicated for most causes of bacterial meningitis, except for meningococcus (droplet precautions) and tuberculosis meningitis (airborne precautions). Electrolyte imbalances seen in bacterial meningitis and their treatment are discussed in Chapter 108 Renal and Electrolyte Emergencies.
TABLE 102.6
USUAL RANGES FOR CEREBROSPINAL FLUID PARAMETERS
Herpes Simplex Virus, Neonatal
CLINICAL PEARLS AND PITFALLS
• Most mothers of infants with HSV infection do not provide a history of HSV, as primary infection can be asymptomatic and vesicular lesions deep in the female genitourinary tract cannot be visualized by the mothers. Thus, a “negative” maternal history of herpes does not rule out herpes in an infant.
• HSV has substantial overlap with bacterial causes of sepsis and meningitis.
• The three main forms of neonatal disease are skin, eye, and mouth (SEM) disease, CNS disease, and disseminated disease.
• HSV should be considered in the differential diagnosis of any febrile neonate with a CSF pleocytosis and in infants with elevated hepatic transaminases or coagulopathy.
• Early recognition of HSV disease and prompt initiation of acyclovir can decrease the substantial morbidity and mortality in infants.
Current Evidence
HSV has three major manifestations in the neonatal period. HSV genital lesions will be described in the section on sexually transmitted infections (STIs). It is estimated that 45% of adults in the United States are seropositive for HSV-1 and 16% for HSV-2. Both viruses can cause oral or genitourinary infection, but approximately 75% of neonatal HSV disease is caused by HSV-2. Neonatal HSV is thought to complicate 1 in 3,200 deliveries, resulting in approximately 1,500 cases per year in the United States. Risk factors for transmission to neonates include primary maternal infection; vaginal delivery; prolonged rupture of membranes; HSV-2; and use of fetal scalp electrodes. The risk of neonatal HSV is highest during the primary infection in the mother, as viremia is often higher than with recurrent infections, and an effective immune response has yet to be mounted. However, 75% of mothers of HSV-infected neonates did not report a history of herpes, as primary infection can be asymptomatic. As such, the lack of maternal history of HSV should not provide false reassurance to the PEM clinician.
TABLE 102.7
EMPIRIC ANTIBIOTIC THERAPY FOR SUSPECTED ACUTE BACTERIAL MENINGITIS
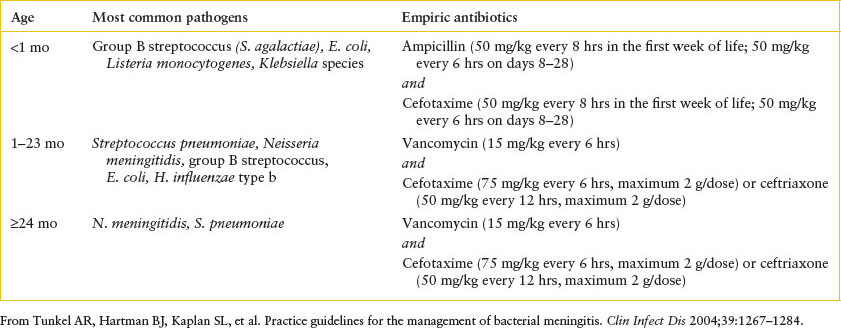
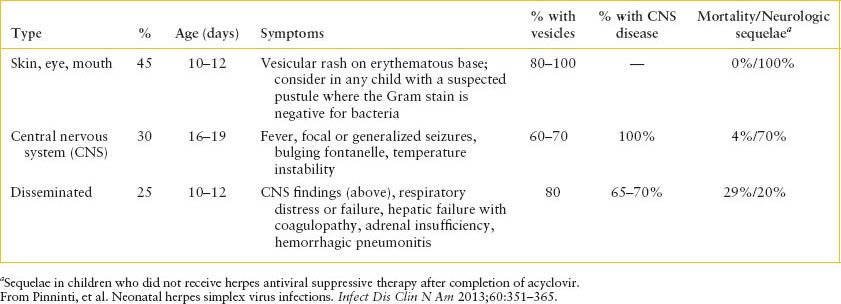
TABLE 102.8
MANIFESTATIONS OF NEONATAL HERPES SIMPLEX VIRUS INFECTIONa
Goals of Treatment
The goal of treatment of neonatal HSV is the rapid recognition and initiation of acyclovir promptly. Clinical outcomes include time to antiviral therapy and neurologic sequelae.
Clinical Considerations
Clinical recognition: There are three manifestations of HSV in the neonatal period: SEM disease, seen in approximately 45% of cases; CNS disease, seen in 30%; and disseminated disease, seen in 25%. The most common clinical and laboratory presentations are described in Table 102.8. In utero infection rarely is seen (1 in 300,000 deliveries) and children are symptomatic at birth with cutaneous, ophthalmologic, and neurologic findings. There is substantial overlap between the three most common disease entities, as many children with vesicles have more invasive disease, and many children with disseminated disease have CNS involvement. Children with isolated SEM disease have the best prognosis, with few reported cases of death or neurologic sequelae. While the mortality rate for CNS disease is low, most children have neurologic sequelae. In contrast to older patients, in whom HSV has a tropism for the temporal lobes, neonatal CNS HSV infection often involves multiple portions of the brain. Children with disseminated disease present septic, and require multiorgan system support, as they often have severe synthetic hepatic dysfunction resulting in coagulopathy and can develop HSV pneumonitis, which is often hemorrhagic. Adrenal involvement is common; as such, the provider should consider hydrocortisone for children with suspected HSV disease. The most severe disease typically occurs in children who never develop a vesicular rash, as the diagnosis often is delayed in these children. As such, the absence of a rash should not lead the clinician to eliminate HSV from the differential diagnosis.
Triage considerations: Febrile and hypothermic neonates should be evaluated promptly for HSV in addition to bacterial infections. The clinician’s index of suspicion for HSV should be increased in the following circumstances: vesicles; seizures or abnormal neurologic examination; CSF pleocytosis; or evidence of hepatic dysfunction, including coagulopathy.
Clinical assessment: The diagnosis is confirmed by isolation of HSV via culture or polymerase chain reaction (PCR). Surface cultures should be obtained from the conjunctivae, nose, mouth, and anus, in addition to cultures being obtained from vesicles. In the latter instance, the swabs should be rubbed against the base of the vesicle, as opposed to aspirating fluid from the vesicle itself. HSV PCR of the CSF is more sensitive (90% to 100%) than viral culture of the CSF, but the sensitivity is lower early in the disease process. As such, a negative HSV CSF PCR does not rule out CNS involvement. If the index of suspicion for HSV disease is high, it is reasonable to repeat PCR testing prior to stopping therapy. PCR (qualitative or quantitative) can also be obtained from the blood; this is of particular utility in children who are coagulopathic or have other clinical features which make LP difficult. HSV serologies are not useful in the acute setting. In addition to routine laboratory evaluation and cultures, ALT, prothrombin time, and partial thromboplastin time should be obtained. Obtaining a serum glucose is also useful, as hepatic dysfunction can result in an inability to mobilize glycogen stores and result in hypoglycemia.
Management: Neonates with suspected HSV should receive parenteral acyclovir (20 mg per kg every 8 hours). Children in whom ocular disease is present should be promptly evaluated by an ophthalmologist and receive topical antiviral therapy, such as 1% trifluridine (one drop every 6 hours) to the affected eye(s), in addition to parenteral acyclovir. Neonates, especially those with disseminated disease, are likely to require blood products and fresh frozen plasma to treat coagulopathy. Many of the considerations noted in the section on bacterial meningitis also are applicable for HSV meningoencephalitis. Standard and contact precautions (if vesicles are present) should be used for children with suspected HSV disease.
Meningitis, Aseptic
Both infectious and noninfectious processes can produce aseptic meningitis (Table 102.9). The most common cause is viral meningitis. In Lyme-endemic regions, clinicians should also suspect Borrelia burgdorferi as a cause of aseptic meningitis. The signs and symptoms of aseptic meningitis mimic those of acute bacterial meningitis, but alterations in level of consciousness or focal neurologic deficits are rarer than in bacterial meningitis. Initial laboratory evaluation should parallel that described for bacterial meningitis. Enteroviral PCR should be sent on the initial CSF. Ill-appearing children or infants in the first 1 to 2 months of life should have HSV PCR on the CSF and serum sent as well (see HSV meningoencephalitis section) and acyclovir (20 mg per kg every 8 hours for children 0 to 3 months; 15 mg per kg every 8 hours for children >3 months of age) should be initiated. Most patients need no further tests, but in atypical situations, consideration should always be given to nonviral causes that may mandate additional diagnostic steps or specific therapy. If tuberculosis is suspected based on family contacts, a low CSF glucose with lymphocytic predominance, or pulmonary findings, then a Mantoux tuberculin skin test (TST) and chest radiograph are useful for confirmation. In endemic areas, serologic studies for Lyme disease and antibiotic therapy may be indicated based upon the exposure history and time of year. A CT scan provides essential information about patients with symptoms or signs of parameningeal infection, HSV encephalitis, or CNS tumors and hemorrhages. Immunosuppressed patients develop infections with a wide variety of unusual bacteria, fungi, and parasites that can be identified in many cases with appropriate examination and culture of the CSF (e.g., India ink and acid-fast stains, cryptococcal antigen testing, fungal and mycobacterial cultures).
TABLE 102.9
CAUSES OF ASEPTIC MENINGITIS
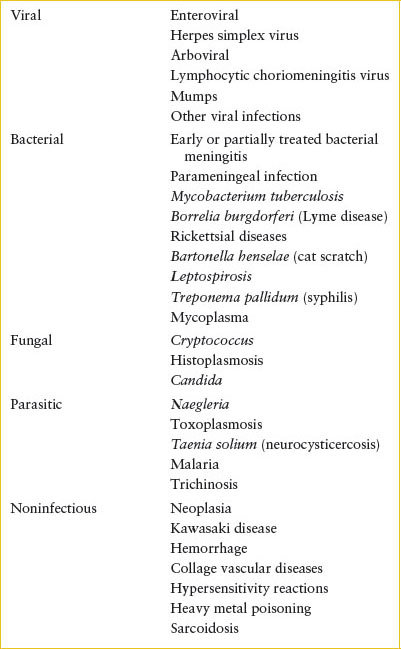
Because the CSF findings in aseptic meningitis overlap those in bacterial infections, hospital admission is usually warranted until the CSF culture results are available. However, the experienced clinician may choose to follow the older child as an outpatient if the family is reliable and nonviral causes (e.g., Lyme disease, tuberculosis, cryptococcosis) are clinically unlikely. To guide clinicians, the Bacterial Meningitis Score has been derived and validated to identify children at very low risk (negative predictive value 99.7%) for bacterial meningitis. Low-risk features are negative CSF Gram stain; CSF absolute neutrophil count (ANC) <1,000 cells/μL, CSF protein <80 mg per dL, peripheral ANC <10,000 cells per mm3, and no seizures at or prior to presentation. Additionally, a positive rapid enteroviral PCR may support outpatient management if available and the patient is clinically well.
Encephalitis and Meningoencephalitis
Encephalitis is an inflammation of the brain that can occur with or without associated meningeal irritation; the former is termed meningoencephalitis, but the terms will be used interchangeably in this section. The clinical manifestations can overlap with those of meningitis. The etiologies most commonly associated with encephalitis are listed in  e-Table 102.2; however, an etiology is found in only a small fraction of children and adults with encephalitis. In circumstances where etiologies are found, almost 70% are viral (most commonly enterovirus, followed by HSV and Epstein–Barr virus [EBV]) and approximately 20% are bacterial. In the last two decades, it has been recognized that several arboviruses endemic in the United States can cause encephalitis. These viruses, which include West Nile, St. Louis, La Crosse, and the equine encephalitides, are termed arboviruses because they are arthropod-borne viruses, not because they share phylogenetic characteristics. The clinical manifestations include altered consciousness or behavioral changes, seizures, hemiparesis, or ataxia, often with nausea and vomiting. Fever is not uniformly present. Postinfectious cases can have associated demyelination in the absence of acute signs of infection; most cases of brainstem encephalitis are postinfectious. The differential diagnosis of encephalitis includes ingestion, metabolic disorders, structural lesions (masses, bleeds, emboli), acute demyelinating encephalomyelitis (ADEM), and autoimmune encephalitis (NMDAR). One diagnostic approach to the child with suspected encephalitis is listed in Table 102.10, realizing that repeated history taking may be necessary to elucidate all exposures a child may have had. Children with encephalitis should be started on acyclovir (20 mg per kg every 8 hours) pending HSV PCR, as this is one of the few treatable causes of encephalitis. If a CSF pleocytosis exists, empiric initiation of parenteral antibiotics (e.g., vancomycin [15 mg per kg every 6 hours] and cefotaxime [75 mg per kg every 6 hours; maximum: 2 g per dose]) is reasonable pending bacterial culture results. Consideration should be given to admission of these patients to intensive care unit settings for closer monitoring given concerns for changes in the ability to protect the airway, increased intracranial pressure, or electrolyte imbalances. Standard precautions are recommended for most forms of encephalitis.
e-Table 102.2; however, an etiology is found in only a small fraction of children and adults with encephalitis. In circumstances where etiologies are found, almost 70% are viral (most commonly enterovirus, followed by HSV and Epstein–Barr virus [EBV]) and approximately 20% are bacterial. In the last two decades, it has been recognized that several arboviruses endemic in the United States can cause encephalitis. These viruses, which include West Nile, St. Louis, La Crosse, and the equine encephalitides, are termed arboviruses because they are arthropod-borne viruses, not because they share phylogenetic characteristics. The clinical manifestations include altered consciousness or behavioral changes, seizures, hemiparesis, or ataxia, often with nausea and vomiting. Fever is not uniformly present. Postinfectious cases can have associated demyelination in the absence of acute signs of infection; most cases of brainstem encephalitis are postinfectious. The differential diagnosis of encephalitis includes ingestion, metabolic disorders, structural lesions (masses, bleeds, emboli), acute demyelinating encephalomyelitis (ADEM), and autoimmune encephalitis (NMDAR). One diagnostic approach to the child with suspected encephalitis is listed in Table 102.10, realizing that repeated history taking may be necessary to elucidate all exposures a child may have had. Children with encephalitis should be started on acyclovir (20 mg per kg every 8 hours) pending HSV PCR, as this is one of the few treatable causes of encephalitis. If a CSF pleocytosis exists, empiric initiation of parenteral antibiotics (e.g., vancomycin [15 mg per kg every 6 hours] and cefotaxime [75 mg per kg every 6 hours; maximum: 2 g per dose]) is reasonable pending bacterial culture results. Consideration should be given to admission of these patients to intensive care unit settings for closer monitoring given concerns for changes in the ability to protect the airway, increased intracranial pressure, or electrolyte imbalances. Standard precautions are recommended for most forms of encephalitis.
TABLE 102.10
EVALUATION OF A CHILD WITH SUSPECTED ENCEPHALITIS OR MENINGOENCEPHALITIS

Other CNS Infections
Goals of Treatment
The goal is to rapidly identify infections which may result in intracranial extension, and to recognize that the empiric antibiotic selection in these cases must include antibiotics that are both bactericidal and achieve adequate CNS penetrance.
CLINICAL PEARLS AND PITFALLS
• The most common comorbidity in children with brain abscesses is congenital heart disease.
• Staphylococci and streptococci are the most common organisms isolated.
• One common regimen to treat suspected CNS invasion from contiguous structures is the combination of vancomycin, ceftriaxone or cefotaxime, and metronidazole, all at meningitic doses.
Brain Abscesses
Brain abscesses can result from contiguous spread from head and neck infections (e.g., mastoiditis, sinusitis, odontogenic) or from direct seeding from septic emboli, most commonly in children with congenital heart disease. The latter remains the most common risk factor for pediatric brain abscesses. The most common organisms are streptococci (aerobic and anaerobic streptococci, GAS, and pneumococcus) and S. aureus, followed by fungal (primarily Aspergillus) and Enterobacteriaceae. Early symptoms are nonspecific and can include fever, malaise, vomiting, and headache. The most common signs are focal neurologic deficits, papilledema, meningeal signs, hemiparesis, and ataxia, although symptoms will vary by abscess location (cerebral hemisphere is the most common location) and size. Mental status changes are late signs with ominous prognoses. LP rarely yields an organism, and blood cultures infrequently are positive. ED-based diagnosis can be made by contrast CT of the brain, although magnetic resonance imaging (MRI) will better delineate brainstem and cerebellar abscesses. Early neurosurgical intervention is critical. Empiric antibiotics should be broad-spectrum antibiotics with CNS penetrance covering staphylococci, streptococci, and anaerobes. One regimen would be vancomycin, cefotaxime, and metronidazole, all at meningitic doses. Standard precautions should be used.
Sinusitis
Sinusitis is an inflammation of the paranasal sinuses. While the ethmoid and maxillary sinuses are present at birth, the frontal and sphenoid sinuses do not develop until children are school aged. The most common etiologies mimic those causing acute otitis media and include pneumococcus, nontypeable H. influenzae, Moraxella, and GAS. The most common signs and symptoms of acute sinusitis are listed in  e-Table 102.3. Children with chronic sinusitis can have milder, more indolent symptoms, such as cough that is often worse when the child is supine and rhinorrhea; pyrexia is less common in this group of children, and physical examination often is normal. The diagnostic criteria are summarized in
e-Table 102.3. Children with chronic sinusitis can have milder, more indolent symptoms, such as cough that is often worse when the child is supine and rhinorrhea; pyrexia is less common in this group of children, and physical examination often is normal. The diagnostic criteria are summarized in  e-Table 102.4. Complications of sinusitis include orbital cellulitis, brain abscess, epidural or subdural empyema, and cavernous sinus thrombosis.
e-Table 102.4. Complications of sinusitis include orbital cellulitis, brain abscess, epidural or subdural empyema, and cavernous sinus thrombosis.
Most sinusitis is managed solely with medical therapy in the outpatient setting. Amoxicillin (80 to 90 mg/kg/day) remains the mainstay of therapy; a 10-day course is recommended for most cases of uncomplicated acute sinusitis. Two- to 3-week courses may be needed for chronic sinusitis or for immunocompromised or chronically ill children with acute sinusitis. Inpatient therapy should be considered for toxic-appearing children, those with facial swelling, or children with suspected or confirmed intracranial extension. The AAP recommendations for sinusitis treatment are summarized in  e-Table 102.5. Standard precautions should be used.
e-Table 102.5. Standard precautions should be used.
Mastoiditis
Mastoiditis, an infection of the mastoid air cells, is a rare complication of acute otitis media. The most common organism is pneumococcus, followed by S. aureus, GAS, and Pseudomonas; historically, Hib has also been implicated. The most common signs are fever, ear proptosis, and postauricular redness, pain, and swelling; the tympanic membrane is usually erythematous and bulging. Complications can include intracranial extension of the abscess, damage to the facial nerve, labyrinthitis, bacteremia, and osteomyelitis. The diagnosis is confirmed by CT of the temporal bone. Tympanocentesis cultures reflect etiology of mastoiditis in approximately 50% of cases. Treatment is a combination of medical and surgical management. Empiric antibiotics should target streptococci and staphylococci. In regions where MRSA and penicillin-resistant pneumococci are common, a reasonable regimen would include vancomycin and a third-generation cephalosporin. Standard precautions should be used.
Orbital Cellulitis
Orbital cellulitis is an infection posterior to the orbital septum caused primarily by S. aureus, streptococci, pneumococcus, and nontypeable H. influenzae. The most common signs are fever, proptosis, limited ocular range of motion, pain with eye motion, chemosis, and an afferent pupillary defect. Blood cultures should be obtained; LP should be considered for young infants and for children with signs of meningitis. Contrasted CT of the orbit and brain confirms the diagnosis and allows for evaluation for intracranial extension, which would alter antibiotic management. Many children with uncomplicated orbital cellulitis are managed medically; however, early consultation with surgical subspecialists would be advised. Empiric antibiotic selection should cover the same organisms as for mastoiditis; if intracranial extension is not evident on CT, substitution of vancomycin for clindamycin can ease the transition of a child from parental to an entirely oral regimen after clinical improvement even if an isolate is not recovered. Indications for operative management include intracranial extension, visual loss, or optic nerve dysfunction. Standard precautions should be used.
Botulism
Botulism is a neurotoxic disorder caused by Clostridium botulinum that causes a descending flaccid paralysis. The most common pediatric manifestation is infantile botulism, most common in children under 6 months of age, caused by ingestion of spores; botulism is the reason that honey is not recommended for infants. Affected infants have decreased movement, bulbar nerve palsies, expression-less facies, loss of head control, and descending hypotonia. Diagnosis is confirmed by isolation of toxin or spores from stool. The mainstay of treatment is supportive therapy; intubation may be necessary. Infants with botulism should immediately receive botulism immune globulin (BabyBIG) intravenously. Antibiotics are not indicated for infantile botulism, and aminoglycosides may worsen the toxin’s paralytic effects. Older patients with wound botulism after penetrating or crush trauma should receive penicillin or metronidazole after receiving an equine-derived Heptavalent Botulinum Antitoxin (HBAT). Standard precautions should be used.
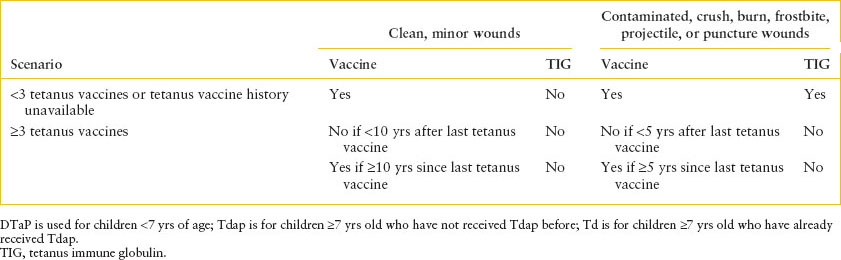
Tetanus
Tetanus is caused by another neurotoxin in the Clostridium family, Clostridium tetani. Spores are ubiquitous in the environment and can contaminate wounds of unimmunized or underimmunized persons. Neonatal tetanus is most common in developing nations where infants are unprotected because of the lack of maternal immunity. Local tetanus refers to muscle spasms in areas contiguous to the wound, and can result in generalized tetanus (lockjaw), with trismus, risus sardonicus, and generalized muscle spasming. The differential diagnosis includes hypocalcemia and drug reactions. Tetanus is a clinical diagnosis; culture yield is poor. Treatment is tetanus immune globulin (TIG), with some infiltrated around the wound and the rest administered intramuscularly. Metronidazole (preferred) or penicillin for 10 to 14 days also is needed. The recommendations for ED management of tetanus prophylaxis are described in Table 102.11. It is important that the ED physician asks about tetanus vaccination, as opposed to assuming that children are up-to-date on this immunization; a recent national surveillance study found that only 82% of toddlers were appropriately immunized against tetanus. Standard precautions should be used.
TABLE 102.11
TETANUS PROPHYLAXIS FOR PATIENTS WITH WOUNDS OR BITES
NECK INFECTIOUS EMERGENCIES
Cervical Lymphadenitis
CLINICAL PEARLS AND PITFALLS
• The most common organisms causing cervical lymphadenitis are staphylococci and streptococci. However, thorough travel and exposure histories should be taken to evaluate for less common etiologies.
• Signs of inflammation also can help differentiate among the causes of localized infectious lymphadenopathy. Nontender adenopathy should lead the clinician away from most pyogenic causes, and should increase the index of suspicion for viral upper respiratory infections (URIs) or mycobacterial disease, depending upon the duration of illness.
Current Evidence
Cervical lymphadenitis is a bacterial infection of the lymph nodes in the neck. This condition must be distinguished from lymphadenopathy, an enlargement of one or more lymph nodes that occurs with viral infections, or as a reaction to bacterial disease in structures that drain to the nodes. The most common etiologies are listed in  e-Table 102.6 (see also Chapter 42 Lymphadenopathy).
e-Table 102.6 (see also Chapter 42 Lymphadenopathy).
Goals of Treatment
Clinical outcomes for children with lymphadenitis include limiting the use of CT among patients with uncomplicated bacterial lymphadenitis.
Clinical Considerations
Clinical recognition: The child with cervical lymphadenitis is usually noted to have swelling in the neck. If sufficiently old, he or she will complain of pain. Fever occurs only occasionally, more often in children younger than 1 year. The infected node may vary in size from 2 cm to more than 10 cm. Initially, it has a firm consistency, but fluctuance develops in about 25% of the infected nodes. The skin overlying the node becomes erythematous, and there may be associated edema. Children with nontuberculous mycobacterial infections may have nontender adenopathy with violaceous discoloration of the overlying skin.
Triage considerations: Children with lymphadenitis should be promptly assessed for deep neck infections and for infections that may affect the airway. Lymphadenitis should be considered in the differential diagnosis of a child with a painful neck mass. Associated toxic appearance or pain out of proportion to the examination may imply a deeper extension of the infection and demand emergent treatment and surgical consultation.
Clinical assessment: The WBC count is usually normal but may be elevated in the younger, febrile child. Aspiration of the node often identifies the organism by both Gram stain and culture, even if fluctuance is not appreciated. Children with infections from Mycobacterium tuberculosis usually react to the TST and may have findings compatible with tuberculosis seen on chest radiograph. Complications of bacterial adenitis are unusual. Organisms such as S. aureus and group A streptococci (GAS) can spread locally if unchecked. A draining sinus tract may develop in untreated children with atypical mycobacterial adenitis. Recurrence of infection suggests a local anatomic abnormality (e.g., branchial cleft cyst or thyroglossal duct cyst) or immunocompromising conditions such as chronic granulomatous disease.
Management: If the node is fluctuant, aspiration provides useful etiologic information and speeds the rate of resolution. Children who fail to respond to empiric antibiotic therapy and children with tuberculosis risk factors who present with adenitis should have a TST placed. Children with cervical adenitis who are otherwise healthy should receive an antibiotic effective against S. aureus and the GAS. While clindamycin (10 mg/kg/dose three times daily; maximum: 600 mg per dose) has activity against both pathogens, trimethoprim-sulfamethoxazole (TMP-SMZ) will not offer GAS coverage. The decision about which oral antibiotic to select depends on the level of methicillin-resistant S. aureus (MRSA) in a community. Indications for inpatient admission and parenteral antibiotics include: toxic appearance; young age (<3 months); immunocompromised host; suspicion of deeper neck extension; development of a draining sinus track; or failure to improve with outpatient oral antibiotic therapy. For these children, clindamycin (10 to 13 mg per kg every 8 hours; maximum: 600 mg per dose) or vancomycin (15 mg per kg every 8 hours; maximum: 2 g per dose) offers alternatives in the face of penicillin and/or cephalosporin allergy or in geographic areas where coverage for MRSA must be considered. Standard precautions should be used unless children have draining lesions (in which case contact precautions should be used) or if children are suspected of having tuberculosis lymphadenitis (in which case airborne precautions should be used until pulmonary involvement is excluded).
Other Neck Infectious Emergencies
Lemierre’s Syndrome
Lemierre’s syndrome refers to a deep neck abscess with a contiguous septic thrombophlebitis of the internal jugular and septic pulmonary emboli. The most common cause historically has been Fusobacterium necrophorum; in recent years, S. aureus has been the most common etiology. Examination is notable for a tender cord in the lateral neck and dyspnea is common as the number of septic pulmonary emboli increases. The diagnosis can be made by CT neck with contrast-showing flow voids in the jugular; apical cuts through the lungs on neck CT may show the embolic lesions. Children with suspected Lemierre’s should receive broad-spectrum antibiotics covering both MRSA and anaerobes (e.g., vancomycin plus metronidazole).
Cat-scratch Disease
Cat-scratch disease, caused by Bartonella henselae, is caused by cat scratches or cats licking abraded skin or from the bite of infected cat fleas. These most commonly occur to the upper extremities and result in tender, fluctuant axillary or epitrochlear lymphadenitis, but cervical adenopathy can be seen if scratches or bites occur to the face. In some pediatric series, Bartonella and EBV are the most common causes of fever of unknown origin. Diagnosis is based on history (cat, especially kitten, exposure), examination (slowly healing papule at inoculation site), and serologies. While most lesions will resolve in 1 to 2 months, antibiotics may decrease symptom duration. Optimal antibiotics are unclear; rifampin, azithromycin, TMP-SMZ, and fluoroquinolones have all been utilized. Standard precautions should be used.
RESPIRATORY TRACT INFECTIOUS EMERGENCIES
Upper Respiratory Tract Infectious Emergencies
Goals of Treatment
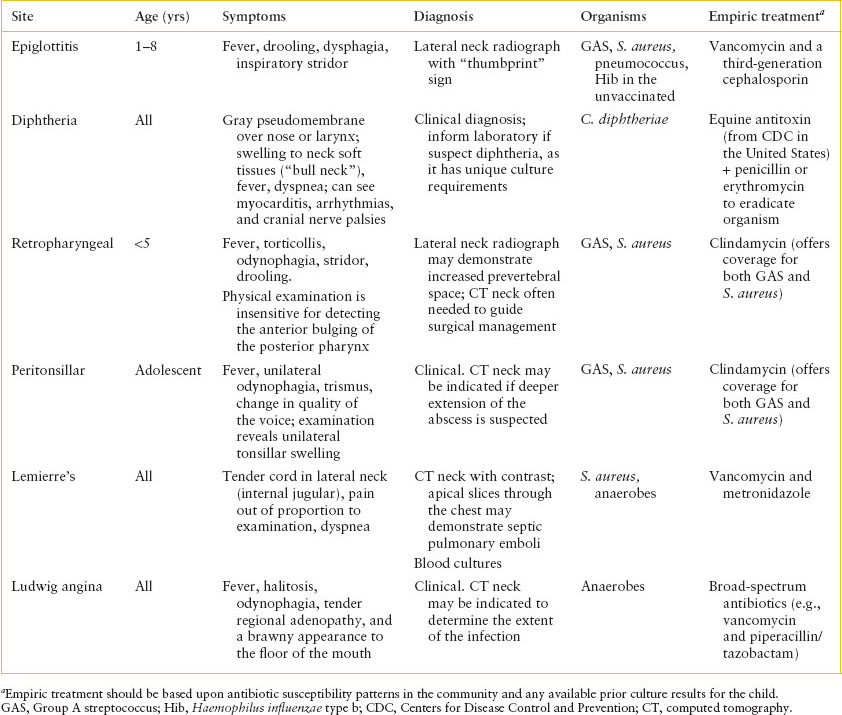
Infections in the neck can rapidly enter other tissue planes that can result in spread to contiguous structures, including compromising the airway or neck vasculature. Clinicians need to be cognizant that while most upper airway infections are relatively benign (e.g., pharyngitis), some can progress from benign etiologies to life-threatening complications (e.g., Lemierre’s syndrome). The clinical manifestations, diagnosis, and treatment of upper respiratory tract infections are summarized in Table 102.12.
CLINICAL PEARLS AND PITFALLS
• Many clinical entities can be differentiated on the basis of the child’s age, history, and oropharyngeal examination.
• Airway instrumentation in a child with suspected epiglottitis should be performed in the more controlled environment of the operating room.
• Many upper airway infections are caused by streptococcal and staphylococcal species, simplifying empiric ED antibiotic selection.
Epiglottitis
Epiglottitis is characterized by fever, drooling, dysphagia, and inspiratory stridor in a toxic-appearing child who is not hoarse and does not have a barky cough. The illness is rapidly progressive over hours and is most common in children 1 to 8 years of age. The most common organism historically was Hib, but now S. pneumoniae, Streptococcus pyogenes, and S. aureus comprise many of the cases. Soft tissue lateral neck radiographs can show an enlarged epiglottis (“thumbprint sign”), and visualization demonstrates a swollen, erythematous epiglottis. However, instrumentation is best performed in the operative setting to prevent airway compromise. Immediate involvement of otolaryngology and broad-spectrum antibiotics (e.g., vancomycin and cefotaxime) and attempting to keep the child calm are the mainstays of ED management. Standard precautions should be used.
Retropharyngeal Abscess
RTAs are characterized by nuchal rigidity or torticollis, difficulty swallowing, drooling, stridor, and fever. Anterior bulging of the posterior pharynx can be difficult to appreciate. Children with RTAs usually are preschool aged, and GAS and S. aureus are the most common organisms. A soft tissue lateral neck radiograph can suggest the diagnosis if there is increased prevertebral space; however, a contrast CT neck is better to delineate the anatomy prior to operative intervention. Empiric antibiotics should target streptococci and staphylococci (e.g., clindamycin). Standard precautions should be used.
Peritonsillar Abscess
Peritonsillar abscesses (PTAs) are characterized by unilateral swelling of the tonsils, change in caliber of the voice, trismus, unilateral odynophagia, displacement of the uvula toward the unaffected side, and fever. PTAs are most common in adolescents, and GAS and S. aureus are the most common organisms. The diagnosis is clinical, although in some cases ultrasound can be used to aid the diagnosis and CT may be useful to evaluate for deeper extension of the abscess. Empiric antibiotics should target streptococci and staphylococci (e.g., clindamycin). Standard precautions should be used.
Ludwig Angina
Ludwig angina is a rapidly progressive cellulitis of the floor of the mouth. This can be a complication of dental abscesses, especially of the mandibular molars. Children with Ludwig angina develop fever, halitosis, odynophagia, submandibular lymphadenopathy, and induration to the floor of the mouth. The most feared complication is airway obstruction, but infection into multiple neck planes can result from Ludwig’s. Anaerobes, including microaerophilic (nonpneumococcal, non-group A) streptococcal species, are most commonly isolated. Diagnosis is clinical, but imaging by CT can help evaluate the extent of infection. Antibiotics should not be held pending imaging or other diagnostic evaluation. Surgical consultation and admission to a critical care unit are necessary with strong consideration for early endotracheal intubation. Broad-spectrum coverage for aerobes and anaerobes (e.g., clindamycin, piperacillin/tazobactam) should be considered for this fulminant infection. Standard precautions should be used.
TABLE 102.12
PRESENTATION OF AIRWAY AND NECK INFECTIONS
Pharyngitis
GAS accounts for 15% to 36% of exudative pharyngitis cases in children and adolescents; the majority of the remaining etiologies are viral. Throat swabs should be obtained from both tonsillar pillars and swabs that first touch the tongue should be discarded, as saliva can result in false-negative rapid streptococcal assay results. Rapid streptococcal assays show variable sensitivity based on the experience of the person collecting the specimen. Reported sensitivities range from 60% to 99%; as such, throat cultures should be sent for all children in whom rapid streptococcal assays are negative. The treatment of choice is amoxicillin or penicillin. There are good data behind the use of a single-daily dose of amoxicillin (50 mg per kg daily, maximum: 1 g per day) for 10 days. For children with difficulty swallowing or in whom nonadherence is a concern, a single intramuscular dose of penicillin should be considered (penicillin G benzathine [Bicillin] ≤27 kg: 600,000 units; >27 kg: 1.2 million units). Regimens for penicillin-allergic patients include cephalosporins and macrolides. There are no data suggesting that the use of cephalosporins decreases the risk of relapse or leads to symptoms resolution faster than more narrow-spectrum antibiotics. Amoxicillin-clavulanate offers no advantages over amoxicillin or penicillin, as there have been no GAS isolates found to be resistant to beta-lactams. Approximately 5% of U.S. GAS isolates are resistant to macrolides and 20% to 25% to clindamycin. Standard precautions should be used.
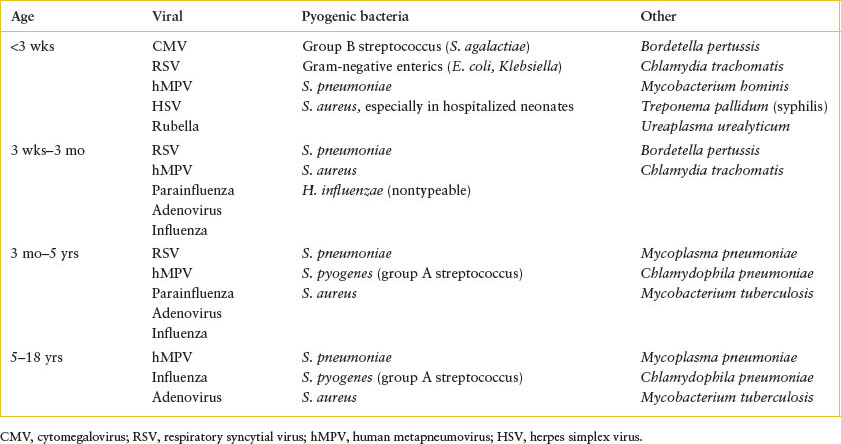
TABLE 102.13
MOST COMMON CAUSES OF PNEUMONIA BY AGE
LOWER RESPIRATORY TRACT INFECTIOUS EMERGENCIES
Lower respiratory tract infections are one of the most common causes of death in children less than 5 years of age in developing nations. The morbidity of these infections in industrialized nations remains high. The following section will review the diagnosis and management of pneumonia and other common lower respiratory tract infections. Tuberculosis is covered separately later in this chapter in the section on infection in returned travelers, reflecting the epidemiology of this disease in industrialized nations.
Pneumonia
CLINICAL PEARLS AND PITFALLS
• The most common causes of community-acquired pneumonia are viral infections.
• Beyond the neonatal period, the most common bacterial cause is pneumococcus.
• Less common, but more severe bacterial causes of pneumonia include S. aureus and GAS.
• While chest radiography can be useful to evaluate for complications of pneumonia, such as empyema or lung abscess, radiographic appearance alone is not useful for differentiating viral from bacterial etiologies.
Current Evidence
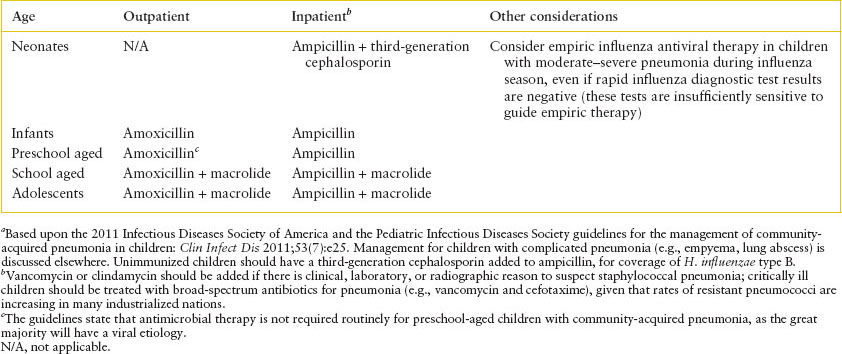
The most common causes of pneumonia in different age groups are listed in Table 102.13. Among the bacteria, S. pneumoniae predominates at every age beyond the newborn period. Hib formerly ranked second to pneumococcus in children 2 months to 3 years of age but now occurs rarely in countries utilizing the conjugate Hib vaccine. S. aureus causes a severe, rapidly progressive but uncommon pneumonia in young children; 60% of these infections occur in the first year of life. GAS is also uncommon but may also be severe, N. meningitidis has been described rarely, and anaerobic bacteria play a role primarily following aspiration.
Goals of Treatment
Early recognition of children with respiratory distress and findings consistent with bacterial pneumonia is ideal. The clinical team should be cognizant of indications for imaging other than chest radiographs, as well as what radiographic patterns may be more consistent with certain pathogens. Clinical outcomes for patients with pneumonia include appropriate antibiotic utilization and indications for admission for treatment.
Clinical Considerations
Clinical recognition: Bacterial pneumonia generally has an abrupt onset with fever, often accompanied by chills. A cough is a common but nonspecific complaint. Younger children may have decreased activity level or appetite. Pleuritic chest pain may be seen. The most common examination finding other than pyrexia is tachypnea. The observation of the child at rest before the examination often provides the key to the diagnosis of pneumonia. A hasty effort at auscultation that disturbs the quiet infant obscures this finding. Grunting respirations in a young child should arouse a strong suspicion of pneumonia. Localized findings, more often seen in the child older than 1 year, include inspiratory rales, decreased breath sounds (sometimes the only abnormality), and less often, dullness to percussion. Patients with lower lobe pneumonia may present with abdominal pain; occasionally, the abdominal findings in pulmonary infections mimic appendicitis. With upper lobe pneumonia, the pain may radiate to the neck, causing meningismus; the diagnosis of pneumonia must, therefore, be considered in the child with nuchal rigidity and normal CSF.
TABLE 102.14
MANAGEMENT OF UNCOMPLICATED COMMUNITY-ACQUIRED PNEUMONIA IN PREVIOUSLY HEALTHY CHILDRENa
Triage considerations: Children with fever and respiratory distress should be evaluated for pneumonia, despite the recognition that only a minority of febrile infants and children with respiratory distress will harbor a bacterial pathogen. Some children with pneumonia will require supplemental oxygen, more advanced airway support, and/or fluid resuscitation.
Clinical assessment: The diagnosis often is made by chest radiograph, which can be falsely negative in dehydrated or neutropenic children. While there are no pathognomonic findings to differentiate viral from bacterial pneumonia, certain patterns in radiographic findings are of use to the PEM clinician. A lobar consolidation is assumed to be of bacterial origin, needing treatment with antibiotics, whereas a minimal, diffuse interstitial infiltrate in a previously healthy toddler suggests a viral infection that can be managed with symptomatic therapy or, in an adolescent, Mycoplasma pneumoniae, calling for treatment with erythromycin or azithromycin. Bilateral involvement, pleural effusion, and pneumatoceles point to more severe disease.
Further laboratory studies are obtained only on specific indications. A WBC count may be helpful in differentiating viral from bacterial disease or in assessing the likelihood of bacteremia in the young child. The rate of occult pneumonia in children with leukocytosis >20,000 per mm3 remains 10% to 15% in the postpneumococcal conjugate vaccine era. An elevated C-reactive protein (CRP) correlates with the bacteremia and lobar infiltrates more closely than the WBC count but is rarely needed. A blood culture is helpful when positive.
The most common complication of pneumonia is dehydration due to decreased intake and increased insensible losses; this is particularly true for young children. Rarely, extensive pulmonary involvement compromises ventilation, leading to respiratory failure. ABGs should be considered for any child with significant respiratory distress or transcutaneous oxygen saturation below 90%. The most common causes of parapneumonic effusions are pneumococcus and staphylococcus. Bacteremia may result in additional foci of infection, including meningitis, pericarditis, epiglottitis, and septic arthritis.
Management: The PEM physician should consider whether or not the child is a candidate for outpatient therapy ( e-Table 102.7). Professional societies have formulated consensus guidelines on which children can be classified as moderate or severe pneumonia and may benefit from intensive care unit care (
e-Table 102.7). Professional societies have formulated consensus guidelines on which children can be classified as moderate or severe pneumonia and may benefit from intensive care unit care ( e-Table 102.8). Empiric antibiotic management is reviewed in Table 102.14. Immunocompromised children should receive broad-spectrum antibiotics, including pseudomonal coverage. The management of children with complicated pneumonia is described elsewhere in the sections on empyema and lung abscess. Standard precautions should be utilized for children with suspected community-acquired pneumonia.
e-Table 102.8). Empiric antibiotic management is reviewed in Table 102.14. Immunocompromised children should receive broad-spectrum antibiotics, including pseudomonal coverage. The management of children with complicated pneumonia is described elsewhere in the sections on empyema and lung abscess. Standard precautions should be utilized for children with suspected community-acquired pneumonia.
Other Respiratory Tract Infectious Emergencies
Tracheitis
Bacterial tracheitis is predominantly caused by S. aureus in the post-Hib vaccine era. It can mimic the presentation of epiglottitis (see above) with a rapid course. While children present with fever and stridor, they are more toxic appearing than children with croup and are in more respiratory distress. Radiographs may reveal tracheal narrowing and direct laryngoscopy may demonstrate a pseudomembrane. The first step in management is to secure the airway; the emergency medicine physician should anticipate that intubation may be difficult; if anesthesiologist or otolaryngology support is available at a facility, consideration should be given to having them at the bedside prior to intubation being attempted. Broad-spectrum antibiotics (e.g., vancomycin and ceftriaxone) should be started and the child should be admitted to an intensive care unit. Standard precautions should be used.
Empyema
Empyemas are purulent pleural effusions that can complicate pneumonia. Empyemas are most commonly seen with S. aureus and pneumococcal pneumonia and increasing incidence rates of staphylococcal pneumonia have been seen in the post-Prevnar era. Gram-negative pathogens should be suspected in immunocompromised hosts, neonates, and patients with indwelling chest tubes. The most common symptoms are fever, shortness of breath, and pleuritic chest pain. The most common examination finding is tachypnea; auscultation can reveal rales or decreased breath sound. Pleural friction rubs are rarely heard in young children. Chest radiography demonstrates blunting of the costophrenic angle. A decubitus or cross-table lateral radiograph can be performed to see if the fluid is free-flowing. Ultrasonography can be very useful to determine if sufficient fluid is present for thoracentesis; for older children, a decubitus fluid layer at least 1 cm thick is considered sufficient to attempt thoracentesis. CT allows for better differentiation between an empyema and lung abscess. Thoracentesis for pleural fluid can be sent for cell count and differential, lactate dehydrogenase (LDH), protein, glucose, and pH in addition to Gram stain and cultures. Cultures that should be obtained include aerobic, anaerobic, and acid-fast cultures. Adenosine deaminase (ADA) should be sent from the pleural fluid if tuberculosis is suspected. The pleural fluid parameters that help differentiate causes of pleural effusion are reviewed in  e-Table 102.9. Some children with empyema will need video-assisted thoracoscopic surgery with debridement; this has been shown to decrease hospital length of stay and fever duration. Empiric antibiotic therapy should target pneumococcus, S. aureus, and GAS. For mildly ill patients, ampicillin and azithromycin treatment for community-acquired pneumonia may be appropriate. For children with risk for staphylococcal disease (e.g., history of staphylococcal disease, presence of pneumatocele), combination therapy with clindamycin and a third-generation cephalosporin is reasonable. Critically ill children should be treated with vancomycin and a third-generation cephalosporin. Anaerobic coverage should be considered for neonates, immunocompromised hosts, associated neck infections (especially with jugular thrombophlebitis), and patients with indwelling chest tubes. Standard precautions are recommended for children with empyema unless tuberculosis is suspected (in which case, airborne precautions should be used) or unless the child has draining skin lesions (in which case contact precautions should be utilized).
e-Table 102.9. Some children with empyema will need video-assisted thoracoscopic surgery with debridement; this has been shown to decrease hospital length of stay and fever duration. Empiric antibiotic therapy should target pneumococcus, S. aureus, and GAS. For mildly ill patients, ampicillin and azithromycin treatment for community-acquired pneumonia may be appropriate. For children with risk for staphylococcal disease (e.g., history of staphylococcal disease, presence of pneumatocele), combination therapy with clindamycin and a third-generation cephalosporin is reasonable. Critically ill children should be treated with vancomycin and a third-generation cephalosporin. Anaerobic coverage should be considered for neonates, immunocompromised hosts, associated neck infections (especially with jugular thrombophlebitis), and patients with indwelling chest tubes. Standard precautions are recommended for children with empyema unless tuberculosis is suspected (in which case, airborne precautions should be used) or unless the child has draining skin lesions (in which case contact precautions should be utilized).
Lung Abscess
Most lung abscesses are polymicrobial and caused by aspiration of oral flora, especially in patients with underlying neurologic disorders. The predominant anaerobes are Bacteroides, Peptostreptococcus, and Prevotella. Anaerobes, S. aureus, pneumococcus, and nontypeable H. influenzae are the most common pathogens identified in otherwise healthy children. Fungal pathogens and Pseudomonas should be considered in immunocompromised children. M. tuberculosis will be discussed separately in the section on travel medicine. The most common symptoms are fever, cough, shortness of breath, and chest pain. Symptoms have often been present for up to 2 to 3 weeks before the child is recognized to have a lung abscess; as a consequence, weight loss is seen in some children, whereas it is an uncommon occurrence for children with community-acquired pneumonia. Auscultation of the lungs is often nonfocal, particularly in young children. The diagnosis usually is made by chest radiograph, which can show a thin- or thick-walled cavity with an air–fluid level. Intrathoracic adenopathy can be found in more subacute causes of lung abscess (e.g., tuberculosis, fungal). CT can be of use if operative intervention is planned to better delineate the anatomy. Leukocytosis with a neutrophilic predominance is common; blood cultures are positive in 10% to 15% of cases. Gram stain of the sputum is rarely useful unless the abscess has ruptured into a bronchus and is communicating with the airway. Percutaneous aspiration or bronchoscopy is more sensitive in yielding a microbiologic diagnosis. Empiric antibiotic coverage should target S. aureus, pneumococcus, and anaerobes. Clindamycin and cefotaxime is one such regimen, with the advantage that it can be readily converted from a parenteral regimen to oral equivalents. However, for toxic-appearing children, or in regions where cephalosporin-resistant pneumococci or clindamycin-resistant staphylococci are commonly seen, vancomycin should be included in the initial regimen. Standard precautions should be used for patients with lung abscesses unless tuberculosis is suspected (in which case, airborne precautions should be implemented).
Pertussis
Pertussis (whooping cough) is caused by Bordetella pertussis or Bordetella parapertussis (the latter being the cause of kennel cough in dogs). A similar clinical syndrome can be caused by adenovirus or Chlamydia trachomatis in infants. There are three clinical stages. The first symptoms are indistinguishable from a viral URI. This catarrhal phase, characterized by a mild cough, conjunctivitis, and coryza, lasts for 1 to 2 weeks. An increasingly severe cough heralds the onset of the second stage (paroxysmal), which continues for 2 to 4 weeks. After a prolonged spasm of coughing often involving 10 or more coughs in succession, the sudden inflow of air produces the characteristic whoop (young infants lack the ability to generate sufficient negative inspiratory pressure and may, therefore, not whoop). During episodes, children can appear to choke, become cyanotic, and appear anxious. Posttussive emesis is common. When not coughing, the child has a remarkably normal history and physical examination, except for an occasional subconjunctival hemorrhage. Young infants can exhibit apnea unrelated to coughing paroxysms. During the third stage (convalescent), the intensity of the cough wanes. At times, pertussis may present as a chronic cough without other signs of infection. The fatality rate for pertussis is approximately 1% for patients in the first month of life and 0.3% for those between age 2 and 12 months. Complications often occur during the paroxysmal stage. The most immediately life-threatening complication is complete obstruction of the airway by a mucous plug, leading to respiratory arrest. Secondary bacterial pneumonia occurs in 25% of children with pertussis and accounts for 90% of the fatalities. Seizures are seen in 3%, and encephalitis in 1%. Sudden increases in intrathoracic pressure can cause intracranial hemorrhages, rupture of the diaphragm, and rectal prolapse.
The white blood count often is elevated, at times with a leukemoid reaction (the latter more common in infants over 6 months of age) and a lymphocytic predominance. Chest radiographs often are normal. The diagnosis is by PCR of nasopharyngeal secretions. Early treatment can reduce symptoms and shorten the clinical course although it is unclear if antibiotics influence course during paroxysmal phase (does still reduce transmission); however, it is important to start antibiotic treatment when pertussis is suspected and prior to confirmatory testing. The preferred treatment is azithromycin (10 mg per kg on the first day, followed by 5 mg per kg on days 2 to 5). Erythromycin (40 mg/kg/day divided every 6 to 8 hours × 14 days, maximum dose: 2 g per day) can also be utilized, though the more frequent dosing interval (every 6 to 8 hours) and longer treatment duration may be associated with reduced adherence. Household and close contacts (even if fully immunized) require chemoprophylaxis with either azithromycin (10 mg per kg daily for 5 days, maximum dose: 500 mg) or erythromycin (same regimen as treatment dose). Contacts who are not fully immunized should also receive a booster dose of the vaccine (DTaP for children <7 years of age, TDaP for children 7 years and above). Receipt of the acellular pertussis vaccine does not obviate the need for postexposure prophylaxis (PEP) for healthcare workers, so strict use of droplet precautions (gloves, mask) is needed for any provider caring for a child with suspected pertussis.
CARDIAC INFECTIOUS EMERGENCIES
Kawasaki Disease
See also:
Chapter 94 Cardiac Emergencies
Chapter 109 Rheumatologic Emergencies
CLINICAL PEARLS AND PITFALLS
• Kawasaki disease (KD) is the most common form of acquired heart disease in children in industrialized nations.
• KD is a clinical diagnosis in a child with fever to 102°F for at least 5 days who has four of five additional symptoms: oral mucosal changes; rash; nonpurulent conjunctivitis; extremity changes; and cervical lymphadenopathy.
• Incomplete KD, where children do not meet all the diagnostic criteria, is more common in young infants, and is a risk factor for the development of coronary artery aneurysms (CAAs).
Current Evidence
KD is a vasculitic condition of unknown etiology that can cause CAAs or ectasia in up to 25% of untreated children. It is the most common form of acquired heart disease in American children. While first described in Japanese children, it occurs in children of all races and ethnicities and is most common in infants and preschool-aged children.
Goals of Treatment
The goal of treatment is to recognize and initiate treatment in children with KD before the 10th day of symptoms, as delayed treatment increases the risk of CAA development.
Clinical Considerations
Clinical recognition: The symptoms of KD are summarized in Table 102.15. Incomplete KD, in which a child does not meet all diagnostic criteria, is more common in infants and rates of CAA are higher in children with incomplete KD. It is important that clinicians ask all caregivers of children with fever of at least 5 days duration about KD symptoms. Not all symptoms may be present at the time of ED presentation. The differential diagnosis of KD is extensive and includes viral infections (especially adenovirus, but can also include EBV, cytomegalovirus [CMV]), scarlet fever, staphylococcal-scalded skin syndrome, TSS, rickettsial diseases (e.g., RMSF), leptospirosis, drug hypersensitivity reactions, and some rheumatologic conditions.
Triage considerations: Children with KD can be intravascularly depleted from insensible losses from several days of high fever. Fluid resuscitation may be needed in the ED. If there is concern for cardiac function based upon examination (e.g., murmur, hepatomegaly), fluid resuscitation should proceed cautiously and early cardiac imaging (or a baseline electrocardiogram) should be obtained.
Clinical assessment: Supporting laboratory criteria are described in Table 102.24.
Management: The treatment for KD is intravenous immunoglobulin (IVIG) at 2 g per kg as a single dose given over 10 to 12 hours. In addition, children should be started on high-dose aspirin (80 to 100 mg/kg/day divided every 6 hours). An echocardiogram should be ordered. A clinician’s threshold for treating should be lower as a child approaches day 10 of fever than it is at day 5. Standard precautions should be used.
Other Cardiac Infectious Emergencies
Goals of Treatment
ED recognition of new cardiac infections is poor, especially in the child without pre-existing structural heart disease. For example, most children with myocarditis are missed at the time of initial ED presentation. Recognition of children at risk for endocarditis, as well as the most common manifestations of myocarditis and pericarditis, can prevent the ED physician from inadvertently worsening cardiac function from rapid fluid resuscitation.
CLINICAL PEARLS AND PITFALLS
• Infective endocarditis is most common in children with structural heart disease, but increased rates of S. aureus endocarditis in children with normal heart valves have recently been described.
• The most common sign of myocarditis is unexplained tachycardia.
• The chest radiograph in a child with pericarditis and a large pericardial effusion may be normal.
• Bedside ultrasound can provide rapid assessment for pericardial effusion and contractility.
Endocarditis
Endocarditis is an infection of cardiac valves most commonly caused by S. aureus, viridans streptococci, the so-called HACEK organisms (Haemophilus noninfluenzae species, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae), and Coxiella (Q-fever). Endocarditis is more common in children with existing structural heart disease. The most common symptoms are fever, chills, fatigue, myalgias, and examination and laboratory findings may include evidence of embolic phenomenon (pulmonary infarcts, intracranial or subconjunctival hemorrhages), Janeway lesions (nontender hemorrhagic macules on palms/soles), immunologic phenomenon (glomerulonephritis, Osler nodes [red, tender raised lesions on palms/soles], or Roth spots [white-centered retinal hemorrhages]). The diagnosis is based on the modified Duke criteria, which combine major criteria (blood culture positivity with an organism known to cause endocarditis; echocardiogram findings) with minor criteria, which include predisposing structural heart disease, fever, and embolic or immunologic phenomenon. ED-based diagnosis involves obtaining several large-volume blood cultures before antibiotics are initiated to optimize culture yield. The laboratory should be instructed to hold the blood cultures for several weeks, as some of the organisms causing endocarditis are fastidious and require long periods to grow. Empiric management in the critically ill child with suspected endocarditis should cover MRSA and Gram-negative organisms (e.g., vancomycin and cefotaxime). Standard precautions should be used.
TABLE 102.15
CLINICAL AND LABORATORY FINDINGS IN KAWASAKI DISEASE
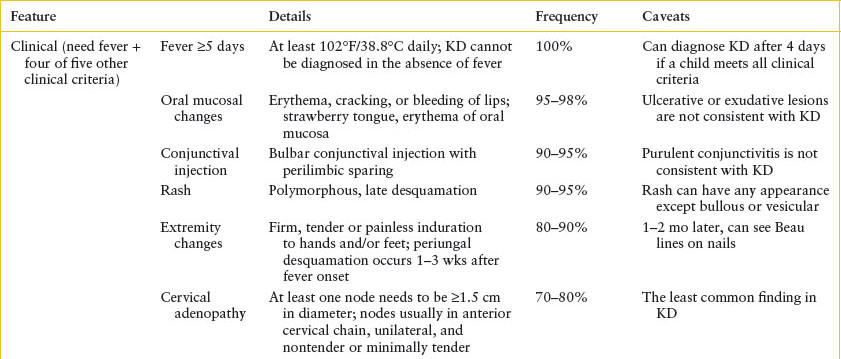
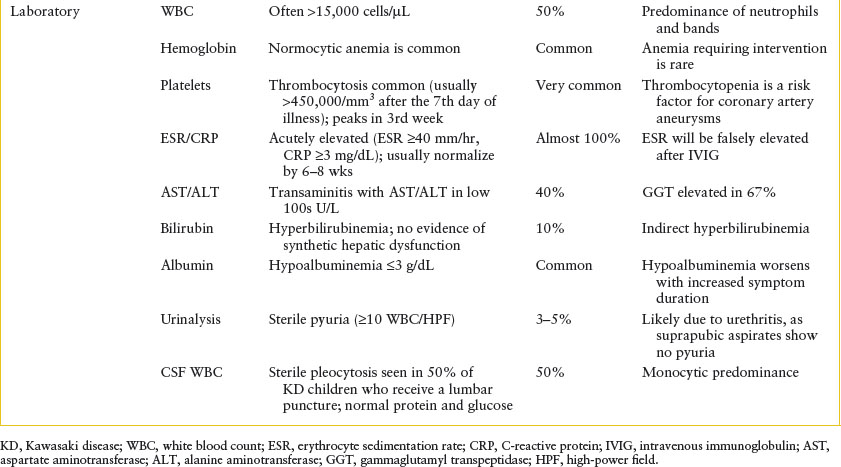
Myocarditis
Myocarditis is a variably painful inflammation of the myocardium primarily caused by viral infections, most commonly enteroviruses, but parvovirus, influenza, parainfluenza, and adenovirus, and other viruses can also cause myocarditis. In children with a consistent travel history, Chagas disease (Trypanosoma cruzi) and parasitic infections can also cause myocarditis. Myocarditis has been found in 15% to 20% of sudden infant death syndrome victims and the same proportion of adolescents who suffer from sudden cardiac death. Early symptoms mimic the nonspecific findings of viral infections; the most common symptoms are shortness of breath, vomiting, poor feeding, rhinorrhea, and fever. Chest pain is more commonly verbalized by older children. The most common examination findings are tachypnea, hepatomegaly, fever, and crackles. Findings may become more obvious after a child receives fluid boluses, emphasizing the importance of serial examinations between fluid boluses. EKGs are abnormal in over 90% of patients; the most common EKG findings are sinus tachycardia, low-voltage QRS complexes, and T-wave inversion. Chest radiographs are abnormal in 60% to 90% of children, most commonly showing cardiomegaly and pulmonary edema. Laboratory evaluation should include troponin, creatine kinase MB, B-type natriuretic peptide (BNP), and early cardiology intervention should be sought. Standard precautions should be used.
Pericarditis is caused by a more heterogeneous group of pathogens (enterovirus being the primary viral etiology, while pneumococcus, meningococcus, S. aureus, and tuberculosis are among the more common bacterial causes). Rheumatologic disorders, uremia, pancreatitis, and other noninfectious etiologies can also result in pericardial inflammation. Symptoms are nonspecific and include fever, cough, shortness of breath, abdominal pain, and vomiting. Chest pain is an early finding and is worst over the precordium, may radiate to the left shoulder, is worse when supine, and better when the child is sitting upright or leaning forward. Examination findings include a pericardial friction rub, muffled heart tones, tachycardia, jugular venous distension (JVD), and narrowed pulse pressures. Pulsus paradoxus, where the systolic blood pressure decreases by >10 mm Hg with inspiration, is concerning for tamponade physiology. Beck triad is pathognomonic for tamponade: JVD, muffled heart sounds, and hypotension. EKGs are abnormal in >90%. Diffuse ST elevation is seen first, followed by ST depression and PR decrease, then normalization of intervals with T-wave inversions. As the pericardial effusion increases, QRS voltages are dampened and there may be evidence of electrical alternans. The chest radiograph often is normal in pericarditis, as it is estimated that an effusion has to be at least 250 mL (adult) before being apparent on plain radiograph. Early cardiology intervention and echocardiogram is critical. Nonsteroidal anti-inflammatory drugs are the mainstay of pericarditis treatment. Children with tamponade physiology may require pericardiocentesis. Standard precautions should be used.
GASTROINTESTINAL INFECTIOUS EMERGENCIES
Gastroenteritis is an inflammation of the alimentary tract that, in its acute form, is overwhelmingly infectious in origin. Viruses are the organisms most commonly found in children with diarrhea in the United States and can be isolated from approximately 30% of patients. In 10% of patients, bacteria are recovered, including Salmonella, Shigella, Campylobacter, Yersinia, and pathogenic E. coli; Aeromonas hydrophila and Vibrio species, such as Plesiomonas shigelloides, are occasional pathogens. Clostridium difficile, which elaborates a toxin, may cause colitis, particularly after the use of antibiotics. Parasitic infestations rarely lead to diarrhea in developed countries. Giardia lamblia and Cryptosporidium should be considered, particularly in outbreaks in daycare centers, and Entamoeba histolytica, among immigrants or travelers from tropical areas; cryptosporidiosis also commonly affects patients with HIV. These topics are covered later in this chapter, in the sections on travel medicine and HIV, respectively. Current diagnostic techniques are unable to identify an etiologic agent in most of the remaining episodes.
Viral hepatitis is covered in Chapter 99 Gastrointestinal Emergencies. Bacterial infections of the liver and bacterial cholangitis, almost exclusively abscesses, are rare in otherwise healthy children; more commonly, they complicate either an anatomic malformation (e.g., biliary atresia) or affect neonates or immunocompromised hosts.
Because calculi in the bile ducts rarely occur before adolescence, cholecystitis occurs much less often in children than in adults. Occasionally, episodes are seen in teenagers or children predisposed to stone formation, as in the chronic hemolytic anemias. Less commonly, salmonellosis, leptospirosis, or KD produces acalculous cholecystitis. These diseases are discussed elsewhere in this chapter.
In childhood, peritonitis almost invariably reflects an intra-abdominal catastrophe that requires surgical intervention. However, the accumulation of ascitic fluid in children with diseases such as nephrosis and cirrhosis allows the development of a primary infection of the peritoneum.
Gastroenteritis—Viral
The most common etiologies of acute gastroenteritis (AGE) in the United States are viral, most commonly noroviruses and rotavirus, which comprise almost 40% of viral AGE in the United States. Rotavirus has been on a major decline since the introduction of routine vaccination. Please also see Chapter 18 Diarrhea
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







