Chapter 9 Incidence of Deep Venous Thrombosis in the Surgical Patient Population and Prophylactic Measures to Reduce Occurrence
An astounding 200,000 to 600,000 people in the United States develop deep venous thrombosis (DVT) and pulmonary embolism (PE) annually. As many as 60,000 to 200,000 individuals will die of PE, making this more deadly in the United States than motor vehicle accidents, breast cancer, or acquired immunodeficiency syndrome (AIDS). DVT has been called a silent epidemic; about half the time the condition causes no symptoms. All too frequently, people die of PE without ever knowing they had a DVT (American Public Health Association, 2003).
Public awareness of DVT increased in the United States after seeing Vice President Dan Quayle experience a DVT (American Public Health Association, 2003). The death of David Bloom, a 40-year-old NBC news correspondent, who died while covering the war in Iraq, heightened awareness even further. His sudden death after experiencing leg cramps with associated pulmonary embolism increased public awareness of DVT and PE (Nutescu, 2007). Despite this growing awareness, approximately two thirds of Americans know nothing about DVT, and more than half of individuals who are aware of DVT are unaware of risk factors, preexisting conditions, or signs and symptoms of DVT (American Public Health Association, 2003).
More than 23 million surgeries are performed in the United States yearly (Agency for Healthcare Research and Quality, 2009), and every surgical patient is at risk for developing DVT or PE. DVT and PE are jointly referred to as venous thromboembolism (VTE). Although all surgical patients are at risk for VTE, many remain at higher risk because extensive operative procedures are being performed on those with greater medical comorbidities and advancing age (Agnelli, 2004). The type of procedure and patient risk factors determine the potential for postsurgical VTE development (Agency for Healthcare Research and Quality, 2009). As many as one half of DVTs associated with surgery start intraoperatively (Kearon, 2003a). Most hospitalized patients who develop symptomatic VTE are diagnosed after discharge from the hospital. What follows is a costly diagnostic and treatment process with the possibility of readmission. Complications are related to anticoagulation and/or long-term morbidity and may result in death (Geerts et al, 2008). Problems associated with VTE could be avoided by the use of simple, cost-effective measures. Nurses participating in a surgical patient’s continuum of care are in a unique position to ensure patient safety by increasing the level of awareness of DVT among patients, physicians, and hospital staff. Aggressively following existing recommendations for VTE prevention can decrease the chance of the deadly complication of pulmonary embolism.
This chapter reviews the background and the incidence of VTE in the surgical patient by surgical specialty, prophylactic measures to reduce occurrence, and nursing implications for practice. Some helpful definitions of common terms can be found in Table 9-1.
| Term | Definition |
|---|---|
| Deep venous thrombosis (DVT) | Blood clot in a deep vein |
| Pulmonary embolism (PE) | Blood clot or fragment of a blood clot breaks loose from the wall of a vein and migrates to the lung, where it blocks a pulmonary artery or one of its branches |
| Venous thromboembolism (VTE) | The manifestation of a DVT or PE |
| Thrombus | A stationary blood clot adhered to a vessel wall |
| Embolus | A thrombus that has separated from a vessel wall and is traveling in the bloodstream to another location |
PATHOPHYSIOLOGY
In 1856 Rudolph Ludwig Karl Virchow, a German pathologist, identified a triad of factors that lead to intravascular coagulation (Figures 9-1 and 9-2). The factors of stasis, vessel wall injury, and hypercoagulability are as pertinent today as they were 150 years ago when identifying the pathogenesis of DVT formation (Crowther and McCourt, 2004; Kucher and Tapson, 2004; Summerfield, 2006 ).
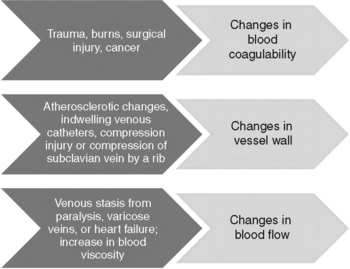
Figure 9-1 Virchow’s triad: coagulation factors.
(From Barnett J, DeCarlo L: Incidence of deep venous thrombosis in the surgical patient population and prophylactic measures to reduce occurrence. Perioper Nurs Clin 3(4):367-382, 2008.)
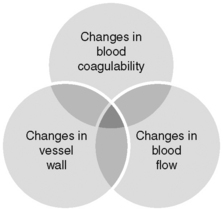
Figure 9-2 Virchow’s triad: relationship between coagulation factors.
(From Barnett J, DeCarlo L: Incidence of deep venous thrombosis in the surgical patient population and prophylactic measures to reduce occurrence. Perioper Nurs Clin 3(4):367-382, 2008.)
DVT occurs when a thrombus consisting of red blood cells, platelets, and leukocytes forms in a large vein. Areas of slow or disturbed blood flow are the most likely location for a thrombus to either partially or completely block circulation. Thrombi can form where a vessel wall injury occurs intraoperatively. Injury can occur from the use of a tourniquet or insertion of an indwelling catheter or during mobilization of vessels to create exposure. Coagulation can be enhanced, or fibrinolysis may be impaired. The result is a hypercoagulable state. Hypercoagulability can be caused by deficiencies in factors that inhibit coagulation (antithrombin III, protein C, protein S) (Woods et al, 2005).
Secondary hypercoagulable states may result from endothelial activation by cytokines, leading to a loss of the normal anticoagulant surface functions of the vessel wall. The vessel wall converts to proinflammatory thrombogenic functions. Vasoconstriction causes venous stasis. The resulting increase in blood viscosity is the precursor to thrombus formation (Woods et al, 2005).
Thrombi may form at any point along the vein wall, but most originate in valve pockets. A thrombus may extend retrograde, further proximally, or expand to completely fill the vessel. Thrombosis is often asymptomatic and resolves spontaneously via the fibrinolytic system through the mechanisms of recanalization, organization, and lysis. Resolution of the thrombosis can be partial or complete (Kucher and Tapson, 2004).
Fifty percent of DVTs start intraoperatively with approximately 50% of these resolving spontaneously within 72 hours. The risk for symptomatic VTE is highest 2 weeks after surgery and remains elevated for 2 to 3 months (Kearon, 2003a). When the condition persists, it can develop into a DVT and lead to a life-threatening PE.
Risk factors for developing a DVT can be divided into two categories: acquired and inherited (Box 9-1). The combination of patient risk factors plus the type of surgery determines risk for developing a DVT. The American College of Chest Physicians (ACCP) has compiled data from prospectively validated evidence-based research studies to define risk groups of low, moderate, high, and highest to recommend prophylaxis either by category of risk or by surgical specialty (Nutescu, 2007).
BOX 9-1 Deep Venous Thrombosis Risk Factors
ACQUIRED RISK FACTORS
Prior major surgical procedure (within 3 months)
Trauma (especially pelvis, hip, or leg fracture)
General versus neuraxial anesthesia
ASA classification status >III
Indwelling venous catheters/CVP/arterial line
Planned postoperative admission to ICU
Immobilization (within 30 days)
Acute infection (urinary, respiratory)
Pregnancy (especially third trimester)
Hormone replacement therapy (highest risk during first year of treatment, 45 to 64 years old)
Long plane trips (>5000 km) and automobile trips
Antiphospholipid antibody syndrome (lupus)
Data from American Public Health Association: Deep vein thrombosis: advancing awareness to protect patient lives, white paper, Public Health Leadership Conference on Deep-Vein Thrombosis, 2003:1–12; Chapman MW et al: Chapman’s orthopaedic surgery, ed 3, Philadelphia, 2000, Lippincott Williams & Wilkins; Ginzberg E et al: Thromboprophylaxis in medical and surgical patients undergoing physical medicine and rehabilitation, Am J Phys Med Rehabil 85(2):159–166, 2006; Kearon C: Duration of venous thromboembolism prophylaxis after surgery, Chest 124:386S-392S, 2003; Kucher N, Tapson VF: Pulmonary embolism. In Fuster V et al, editors: Hurst’s the heart, ed 11, New York, 2004, McGraw-Hill; Race TK, Collier PE: The hidden risks of deep vein thrombosis—the need for risk factor assessment, Crit Care Nurs Q 30(3):245–254, 2007; Summerfield D: Decreasing the incidence of deep vein thrombosis through the use of prophylaxis, AORN J 84(4):642–645, 2006.
Cancer increases the risk for DVT. Intrinsically tumor production can affect coagulation parameters. Extrinsic factors that contribute to the pathogenesis of thromboembolism are indwelling access catheters used for chemotherapeutic agents, the chemotherapeutic agents, and venous stasis occurring because of weakness and decreased ambulation. Pancreatic, lung, gastrointestinal tract, and breast cancers are associated with a higher risk for DVT (Kucher and Tapson, 2004). The Eighth ACCP Conference on Antithrombotic and Thrombolytic Therapy guidelines have been used by The Joint Commission to create performance standards for VTE risk assessment and prophylaxis (Summerfield, 2006; Race and Collier, 2007).
Nutescu (2007) describes a study using a computer-based VTE screening tool for patients on admission to the hospital. An electronic alert is sent to the patient’s physician notifying him or her of the patient’s risk for VTE. The ideal system would go one step further. It not only would identify a patient’s risk for VTE, but would also specify the AACP recommended prophylaxis based on risk factors and type of surgery scheduled. To be successful, a VTE risk assessment tool must be easy to understand and implement. A combination risk assessment tool and prophylaxis order sheet in the patient’s medical record could be completed collaboratively by the nursing staff and the medical staff.
SIGNS AND SYMPTOMS OF DVT AND PE
The clinical manifestations of DVT vary based on the size of the thrombus, the affected vein, and the adequacy of collateral circulation (Woods et al, 2005). The classic symptoms of DVT include swelling, pain, and discoloration of the affected extremity (Ramzi and Leeper, 2004). Calf tenderness, low-grade fever, fatigue, tachycardia, and diaphoresis are also symptoms of DVT (Brunicardi et al, 2004). Physical examination may reveal a palpable cord of thrombosed vein, unilateral edema, warmth, or superficial venous dilation (Ramzi and Leeper, 2004). Because the clinical manifestations of DVT are so variable, objective testing and a thorough history and physical examination should be obtained (Woods et al, 2005). The cardinal signs and symptoms of PE are shortness of breath, pleuritic chest pain, and mental status changes (Brunicardi et al, 2004).
METHODS OF PROPHYLAXIS
Despite risk factors being identified, VTE prophylaxis is underused because of a lack of awareness of DVT risk, variation in the perception of risk factors, and concerns about the risk for bleeding with prophylaxis (American Public Health Association, 2003; Broughton et al, 2007). The most recent recommendations from the American College of Chest Physicians for antithrombotic and thrombolytic therapy were published in 2008 from the eighth ACCP conference (Geerts et al, 2008). The ACCP recommendations for VTE prophylaxis are developed using comprehensive literature searches of evidence-based research. Prevention of VTE falls into two categories: mechanical and pharmacologic prophylaxis.
Mechanical Prophylaxis
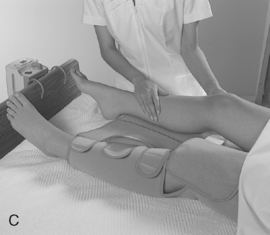
Mechanical measures are simple to implement and do not increase the risk for bleeding. People who are low-risk for developing VTE or have contraindications for the use of pharmacologic measures are good candidates for mechanical prophylactic measures, including early ambulation, foot and ankle exercises, passive range of motion, graduated compression stockings (GCS), and intermittent pneumatic compression (IPC) devices (Kehl-Pruett, 2006). All of these measures improve venous return and reduce venous stasis in leg veins during the immediate postoperative period when patients have decreased mobility because of pain (Pearse et al, 2007). Refer to Figure 9-3 for examples of intermittent pneumatic compression devices.
The advantages of using GCS, IPC, and foot pumps are that they carry no risk for bleeding or drug interactions. The disadvantages are that they require virtually constant use and that compliance is difficult to assess. The evidence of efficacy is limited because of variations in equipment design, and they are often used in combination with pharmacologic treatment. Nevertheless, mechanical methods of thromboprophylaxis are safe and useful adjuncts to pharmacologic approaches (Ginzberg et al, 2006).
Graduated Compression Stockings
Graduated compression stockings may reduce the risk for DVT by multiple mechanisms. Compressing the leg reduces the size of the veins, resulting in increased blood velocity, thereby reducing stasis. Reducing the size of the veins also improves the function of the venous valves and decreases pooling. GCS provide greater compression at the ankle and augment the effect of the calf muscle pump. All of these mechanisms cause a reduction of venous pooling, which can result in an alteration in the levels of clotting factors that may lead to thrombus formation (Mazzone et al, 2008). The use of GCS enhances the protective effect of low-dose unfractionated heparin (LDUH) against DVT by an additional 75% compared with LDUH alone (Geerts et al, 2008).
Proper fit is necessary to prevent a tourniquet effect from GCS that are too tight. They should be removed once a shift for 30 minutes to assess underlying skin (Kehl-Pruett, 2006). Contraindications for using GCS are certain dermatologic diseases, peripheral arterial disease, and diabetic neuropathy. Compression stockings have the potential to cause ischemic necrosis of the legs in patients with peripheral arterial disease (Mazzone et al, 2004).
Intermittent Pneumatic Compression
Intermittent pneumatic compression simulates the effects of walking and weight bearing. Several studies have shown that mechanical devices (continuous passive movement and IPC devices) increase the velocity of blood flow, reduce venous stasis, and reduce the incidence of DVT (Pearse et al, 2007). In addition to the mechanical process of increasing blood flow through the femoral veins, IPC has an antithrombotic effect on the components in the coagulation cascade (Eisle et al, 2007).
Devices vary by site of application, rate of inflation/deflation, pressure amplitude, and chamber inflation sequence (Eisle et al, 2007). Successful use depends on proper sizing and application of the sleeve containing the air chambers. Calf devices inflate and deflate to mimic the calf muscle pump (Crowther and McCourt, 2004). The effect is simulation of the calf muscle stretching rather than simulating muscle contraction. Elongation of the muscles in dorsiflexion of the foot increases the flow of blood in the superficial femoral vein fourfold (Eisle et al, 2007).
Early calf IPC devices used low pressure and slow inflation of a single air bladder. More recent devices have multiple air chambers that produce graduated, sequential, and rapid inflation. The newer technology has been shown to improve hemodynamic response compared with the slower, low-pressure inflation of previous devices (Eisle et al, 2007).
Venous plexus foot pumps mimic the action of walking. The devices intermittently compress and relax the sole of the foot, causing muscle contraction and improving venous blood flow (Crowther and McCourt, 2004). Calf IPC has been proved to be superior to IPC of the foot (Eisle et al, 2007). Calf IPC or venous plexus foot pumps should not be used on patients with acute DVT; peripheral vascular ischemia; large, open wounds; skin grafts; or cancer of the extremity. If compression devices are not applied at the onset of bed rest or in surgery, an ultrasound examination should be performed to rule out DVT before initiating compression therapy (Day, 2003).
Intermittent compression devices are the safest and least invasive intervention used to decrease the incidence of DVT; however, the effectiveness is limited by poor compliance rates. To be effective, they must be used for the duration of bed rest—not just a few hours a day. The factors negatively affecting compliance include the following: doctors, nurses, and patients not understanding the importance of IPC; no formal documentation system for the use of IPC devices; and nurses not noticing if an IPC device is ordered or if it is in good working condition (Kehl-Pruett, 2006; Stewart et al, 2006).
Common patient complaints about IPC include a feeling of warmth or having difficulty sleeping with the continuous inflation and deflation (Kehl-Pruett, 2006). Patients most likely to fail IPC prophylaxis include those with cancer or a past history of DVT and those who are older than 60 years old. This higher-risk group should be considered for more intensive prophylaxis (Clarke-Pearson et al, 2003).
Inferior Vena Cava Filters
The most invasive mechanical prophylactic measure to prevent VTE is the placement of an inferior vena cava (IVC) filter. There are multiple indications for placement of an IVC filter. For example, patients who are not good candidates for chemical anticoagulation but need PE prevention may be considered for IVC placement. Preoperative placement of an IVC filter may be appropriate in patients with a history of DVT or PE who are about to undergo a lengthy neurosurgical procedure and have a risk for postoperative bleeding (Agnelli, 2004; Epstein, 2005). Patients with a prior history of DVT who are undergoing a Roux-en-Y gastric bypass procedure for weight loss are also considered candidates for an IVC filter (Prystowsky et al, 2005).
Pharmacologic Prophylaxis
The clinical importance of administering anticoagulant therapy is to decrease the risk for long-term symptomatic VTE and prevent postthrombotic syndrome (Kearon, 2003b). Anticoagulation therapy includes low-dose unfractionated heparin (LDUH), low-molecular-weight heparin (LMWH), synthetic pentasaccharides, warfarin, vitamin K antagonists, and aspirin.
Bleeding is the greatest concern when choosing anticoagulant therapy as a prophylactic measure to prevent VTE. However, multiple studies have shown no increase in major bleeding with LDUH or LMWH. There is a slightly higher risk for minor bleeding with LDUH compared with LMWH (Broughton et al, 2007).
Heparin
Heparin works as a catalyst, activating and binding to antithrombin III, an endogenous antagonist against several coagulation factors. The main effect of LDUH on the coagulation cascade is blocking thrombin and fibrin formation (Woods et al, 2005; Ginzberg et al, 2006). Dosing is based on weight and requires monitoring of partial thromboplastin time (PTT). Dose adjustments are made based on laboratory results. Low-dose unfractionated heparin can be administered intravenously or subcutaneously depending on whether effects are needed immediately or within approximately 1 hour (Woods et al, 2005; Kehl-Pruett, 2006).
Low-molecular-weight heparin activity is more focused than LDUH. The main effect of LMWH is inhibition of factor Xa, thus interfering with the coagulation cascade one step earlier than the action of LDUH (Ginzberg et al, 2006). The advantage of LMWH is it does not bind to plasma proteins. The result is a more predictable anticoagulant dose (Woods et al, 2005). Low-molecular-weight products are eliminated through the kidneys. Therefore patients who have renal impairment, are obese, or are older adults should be considered at high risk for complication with the use of LMWH (Nutescu, 2007). Patients with a higher risk for bleeding should be started on LMWH rather than LDUH (Kehl-Pruett, 2006). Compared with LDUH, LMWH offers comparable or superior efficacy and a similar or lower risk for bleeding (Ginzberg et al, 2006). Low-molecular-weight heparin (enoxaparin, dalteparin, tinzaparin, reviparin, and nadroparin) can be administered once or twice daily on an inpatient or outpatient basis without coagulation monitoring (Kehl-Pruett, 2006). Low-molecular-weight heparin has a higher acquisition cost but is more cost-effective because it does not require PTT testing (Chapman et al 2000; Ginzberg et al, 2006).
There should be special consideration of patients receiving neuraxial anesthesia (epidural and spinal anesthesia) with the use of LMWH or other heparinoids. These patients have risk for the development of epidural or spinal hematoma, which can lead to paralysis (Morrison, 2006). Although the risk for serious complications is low (Rowlings and Hanson, 2005), the risk is increased when the patient has an indwelling epidural catheter or is receiving concomitant nonsteroidal antiinflammatory drugs, platelet inhibitors, or other forms of anticoagulation (Morrison, 2006). The risk for hematoma versus the benefit of regional anesthesia must be taken into account when considering appropriate patient care (Rowlings and Hanson, 2005). However, the 2003 Consensus Conference of the American Society of Regional Anesthesia (Rowlings and Hanson, 2005) clearly indicates that regional anesthesia can be safely used with LMWH prophylaxis with careful attention to calibration of total daily dose and timing of the initiation and continuance of LMWH in relation to neuraxial anesthesia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree