The Perioperative Period—An Underutilized “Window-of-Opportunity” to Prevent Metastatic Disease
The occurrence of metastatic disease is the leading cause of death in most patients with cancer. Accumulating evidence suggests that the perioperative period, days to weeks before and after surgery, is a critical time frame in which multiple factors profoundly affect initiation, progression, and/or elimination of metastases, providing a critical window of opportunity to prevent metastatic disease. Surgery is the primary life-saving therapeutic approach for many patients with cancer. However, even after successful surgery, minimal residual disease (MRD) in the form of scattered single tumor cells and/or micrometastases are evident in a substantial portion of patients. Additionally, several aspects of oncological surgery have been shown or suggested to promote metastasis, including: (i) increased secretion of stress hormones , ; (ii) local and systemic inflammation , ; (iii) shedding of tumor cells into the circulation , ; (iv) blood transfusions , ; (v) hypothermia ; and (vi) use of specific anesthetic/analgesic agents , , (e.g., intravenous lidocaine and propofol-TIVA are potentially preferred approaches in colorectal and lung cancers). Specifically, these processes act directly on tumor cells and promote their capacity to survive, extravasate, migrate, seed, and release proangiogenic factors and additional progrowth/prometastatic factors while inhibiting a patient’s antimetastatic immune response. These processes are hypothesized to act synergistically on preexisting micrometastases and on isolated tumor cells scattered at the time of surgery, and thereby catalyze cancer recurrence that may not become evident for months or years following surgery when metastases reach a detectable size. For the patient receiving cancer resection surgery, the perioperative period thereby represents a vulnerability to cancer recurrence, which also provides an opportunity for effective intervention.
Clinical examples indicate that the inhibition of prometastatic perioperative processes can improve long-term cancer outcomes, indicating the nonproportional high impact of this short timeframe, specifically: (i) adherence to Enhanced Recovery After Surgery (ERAS) protocols in colorectal cancer (CRC) surgery , ; (ii) enhancing perioperative immunity through use of short preoperative interleukin-2 (IL-2) treatment in colorectal and pancreatic cancer , ; and (iii) perioperative hormone (progesterone) therapy in patients with breast cancer (BC), have all been shown to improve long-term cancer outcomes. Such clinical trials suggest that brief, targeted perioperative therapies could offset perioperative prometastatic processes, ultimately improving cancer survival. Additional therapies under investigation include inhibition of neutrophil extracellular trap (NET) formation and the use of neuroaxial anesthesia to reduce surgical stress. , Taken together, current evidence suggests that short-perioperative interventions (some already part of best practice guidelines , ) may improve patients’ long-term survival following cancer surgery.
As elaborated later, driving many of the prometastatic effects of the perioperative period is the abundant release of inflammatory and stress-related hormones that accompanies surgery. Prominent among these are prostaglandins (PGs) and catecholamines (CAs; epinephrine, EPI; norepinephrine, NE). Both PGs and CAs have been consistently shown in animal models and clinical trials to promote metastasis through their perioperative impact on tumor cells and immunity. The effects of inflammation, a hallmark of cancer, on cancer progression were noted over a hundred years ago, and the deleterious effects of CAs on cancer progression have also been thoroughly documented during the last two decades. , More recently, perioperative care has been dominated by ERAS programs designed to maximize short-term clinical recovery and have been utilized with great success. However, for the patient with cancer, a focus must also be placed on limiting the adrenergic-inflammatory response to reduce perioperative vulnerability to cancer metastasis, thereby improving long-term survival.
In this chapter, we (i) provide evidence for the importance of synergistically addressing inflammatory and adrenergic stress responses to surgery in order to reduce the risk for postsurgical metastatic disease and (ii) discuss how this may be achieved with simple and readily available clinical therapies.
Perioperative Stress-Inflammatory Responses: the Prometastatic Edge of the Sword
During the last three decades, translational , , and clinical research , , has shown that the perioperative secretion of PGs and CAs induced by anxiety, tissue damage, pain, and a variety of surgery-related procedures affects cancer cells directly and further promotes metastasis through their impact on immunity and the cancer microenvironment , , , (reviewed in Horowitz et al., 2015 ). Importantly, PGs and CAs (i) copotentiate each other’s synthesis and secretion and (ii) their impact eventually converges on the same intracellular molecular pathways (e.g., cAMP-PKA), establishing a synergistic inflammatory-stress response (ISR) to surgery. ISRs drive cancer cells’ epithelial-to-mesenchymal transition (EMT), migration, motility, survival, invasiveness, and angiogenesis, as well as suppress anti metastatic immune activities. , The effects of these ISRs, mediated also through PGs and CAs, could transform a life-saving operation into a double-edged sword, excising the malignant tissue but increasing the risk of recurrence.
ISRs Are Initiated Before Surgery
ISRs are known to be induced while anticipating threatening events (e.g., skydiving, public speaking, and surgery). , Specifically, expecting such events was shown to be accompanied by elevated levels of NE, EPI, cortisol, and proinflammatory cytokines such as CRP and IL-6. , Similarly, elevated levels of stress and inflammatory agents are evident a day before surgery. , Thus, the preoperative period is one in which ISRs are already elevated and facilitate prometastatic processes and immune inhibition, suggesting the need to therapeutically address ISRs even before surgery.
Inflammatory and Stress Responses Mutually Potentiate Each Other
Although stress responses and inflammatory responses are triggered separately, they also potentiate each other, creating an integrated ISR. CAs are secreted systemically (EPI) and locally released (NE) in response to sympathetic nervous system (SNS) activation due to stress and/or tissue damage. , Tissue damage also leads to the release of arachidonic acid, which is eventually metabolized by COX enzymes to synthesize PGs. SNS activation, through adrenergic signaling, promotes the metabolism of arachidonic acid and facilitates the synthesis of PGs. In vivo exposure to chronic stress and in vitro exposure to EPI leads to upregulation of COX2 expression in cancer cell lines and in macrophages, , and to increased production of PG-E2 39 (the most abundant PG) and proinflammatory cytokines such as IL-6. Importantly, adrenergic signaling can also lead to changes in lymphatic structure and flow, , and to recruitment of immune cells from the spleen and bone marrow into the circulation, modulating their activity in a manner that may potentiate proinflammatory responses. Peripheral inflammatory processes initiated by PG synthesis and mediated by cytokines crossing the blood–brain barrier can induce central nervous system (CNS) neuroinflammation and is known to increase and sustain adrenergic signaling. In addition, proinflammatory cytokines (e.g., IL-6) can activate nociceptors, leading to local secretion of NE, and sustained pain can induce anxiety and systemic release of EPI. Taken together, inflammatory and adrenergic signaling copotentiate each other in a manner that can lead to a self-perpetuating cycle of increasing inflammatory-adrenergic signaling (see Fig. 9.1 ).

Converging Intracellular Molecular Pathways of PGs and CAs
Although PGs and CAs act on different extracellular receptor systems, they often activate the same intracellular molecular pathways, leading to similar prometastatic consequences. EPI and NE bind to α and β receptors, of which binding to β2 receptors was repeatedly shown to promote many of the prometastatic effects of adrenergic signaling, , but other adrenergic receptors (e.g., β3) were also reported to deleteriously affect metastasis. PGs are synthesized by epithelial, cancer, and immune cells (e.g., macrophages) through the metabolism of arachidonic acid by the constitutively active COX1 and tissue-damage (e.g., surgery)-induced COX2 enzymes. PG and CA receptors are expressed by most immunocytes and epithelial cells and are overly expressed by many tumors. , Both PGs and CAs elevate intracellular cAMP levels and subsequently lead to activation of various prometastatic signaling pathways (e.g., cAMP-PKA, cAMP-EPAC). , Notably, activation of both adrenergic and prostanoid receptors affects similar transcription pathways (e.g., NF-κB, STAT-3, CREB, AP1, GATA1, ETS) through which they exert potent prometastatic effects on cancer and immune cells. , , ,
Overall, during the perioperative period, both PGs and CAs (i) are induced independently and through multiple mechanisms, starting even before surgery; (ii) potentiate the synthesis and release of each other; and (iii) activate similar intracellular prometastatic pathways. Thus combined beta-adrenergic and COX inhibition initiated before surgery could be optimal.
Herein, we will discuss the specific cancer-promoting mechanisms of PGs and CAs and the pharmacological agents currently available to block their effects perioperatively.
Direct Effects of PGs and CAs on Cancer Cells
The activation of the cAMP pathway by PGs and CAs induces prometastatic transcription activity. Specifically, NF-κB stimulation of the Snail, Slug, and TWIST1 transcription factors induces the tumor prometastatic processes necessary for the survival and dissemination of cancer cells. This includes the initiation of EMT and upregulation of HIF-1α, which enhances hypoxic conditioning and facilitates survival of circulating tumor cells with the potential to establish metastasis. Additionally, cAMP-PKA-NF-κB activation leads to tumor secretion of (i) prometastatic and proinflammatory cytokines, such as IL-6, IL-1, IL-8, and TNFα; (ii) proangiogenic VEGF; and (iii) extracellular matrix degrading MMP2 and MMP9. , ,
Importantly, proinflammatory cytokines, such as TNF secreted by tumor cells, lead to activation of NF-κB, thus creating an autocrine positive feedback loop sustaining inflammatory conditions in the tumor microenvironment.
NF-κB is a prominent pathway by which many of the prometastatic effects of CAs and PGs are manifested. It was recently suggested that NF-κB regulation by NE is cell and context specific, such that elevated systemic levels of NE and EPI could potentially suppress inflammation in one context while elevating it in another. Thus the blockade of beta-adrenoceptors may not always suppress inflammation and/or prometastatic processes. A recent review of clinical studies also suggested that the impact of beta blockers is cancer and context specific. This and the fact that both CAs and PGs are simultaneously elevated , during the perioperative period may explain why in some animal models only the combined treatment of nonsteroidal antiinflammatory drugs (NSAIDs) and beta blockers has been effective in reducing postsurgical metastatic burden. , ,
ISRs and Immunity
Both animal and human studies demonstrate that PGs and CAs suppress immunity. , Specifically, CAs recruit immunocytes into the circulation, , and in parallel CAs and PGs induce tumor cell secretion of chemokines , , that attract monocytes into the malignant tissue. Additionally, CAs and PGs directly facilitate polarization of tumor-associated macrophages (TAMs) into the M2 prometastatic phenotype. Importantly, M2-like TAMs were shown to promote extravasation and metastasis and inhibit cell-mediated immunity. Furthermore, TAMs express COX2 and produce PGs, and this can be enhanced by beta-adrenergic signaling. Additionally, macrophages are known to secrete NE in response to LPS stimulation (in vitro) or in hypothermic animals. Hence, adrenergic and prostanoid signaling leads to the recruitment of TAMs into the tumor environment, and TAMs in turn facilitate a proinflammatory prometastatic environment and can potentially secrete PGs and NE in a non-SNS dependent manner. Clinically, this suggests that even patients who do not exhibit anxiety and/or a systemic adrenergic response pre- or perioperatively may still benefit from pharmacological inhibition of CAs and PGs signaling.
ISRs exert extensive immune suppression as most immune cells express receptors for PGs and CAs. , , Binding of PGs and CAs to these receptors was shown to (i) suppress NK cytotoxicity in vitro and in vivo , and reduce the cytotoxicity of T cells, , (ii) shift the Th2/Th1 balance toward Th2 dominance (regarded as prometastatic), (iii) reduce lymphocyte number in critical compartments where circulating cancer cells might be retained (e.g., lung marinating pool), and (iv) inhibit lymphocyte infiltration into tumor tissue. Thus the combined effects of CAs and PGs can lead to immune suppression and can change the systemic and tumor immunocyte milieu in a manner that supports cancer progression. ,
ISRs and the Cancer Microenvironment
CAs and PGs can facilitate prometastatic changes in the tumor microenvironment through a number of mechanisms. Both CAs and PGs can cause immune cells (e.g., macrophages, lymphocytes) to secrete MMP2 and MMP9, leading to extracellular matrix degradation that promotes tumor cell intravasation. , Notably, NE was shown to induce inhibin b A (INHBA) secretion by cancer cells, promoting a cancer-associated fibroblast (CAF) phenotype that can substantially potentiate tumor growth, invasion, and migration. Inflammatory processes through COX signaling can induce platelet activation and aggregation, which provides protection and cloaking to circulating tumor cells, thus promoting their migration capacity. Notably, EPI can also promote platelet activation and aggregation and is routinely used in in vitro studies for experimental activation of platelets. , Further assisting the migration of tumor cells, adrenergic signaling can increase the density of lymphatic vessels and lymphatic flow, as was shown in both animal models and recently in humans. , Such changes in the lymphatic vasculature were shown to be correlated with increased lymphatic presence of tumor cells, increased lymph-node involvement, and increased lung metastases.
Given the abundant release of both CAs and PGs in the perioperative period, preventing the multiple prometastatic effects of ISRs may be especially important during this critical period. The “double-edged sword” of the perioperative period is such that while surgery represents a mainstay of solid tumor cure, the potential exists for perioperative ISRs to promote cancer recurrence and for their blockade to prevent it.
Addressing Perioperative Inflammation and Adrenergic Stress Responses
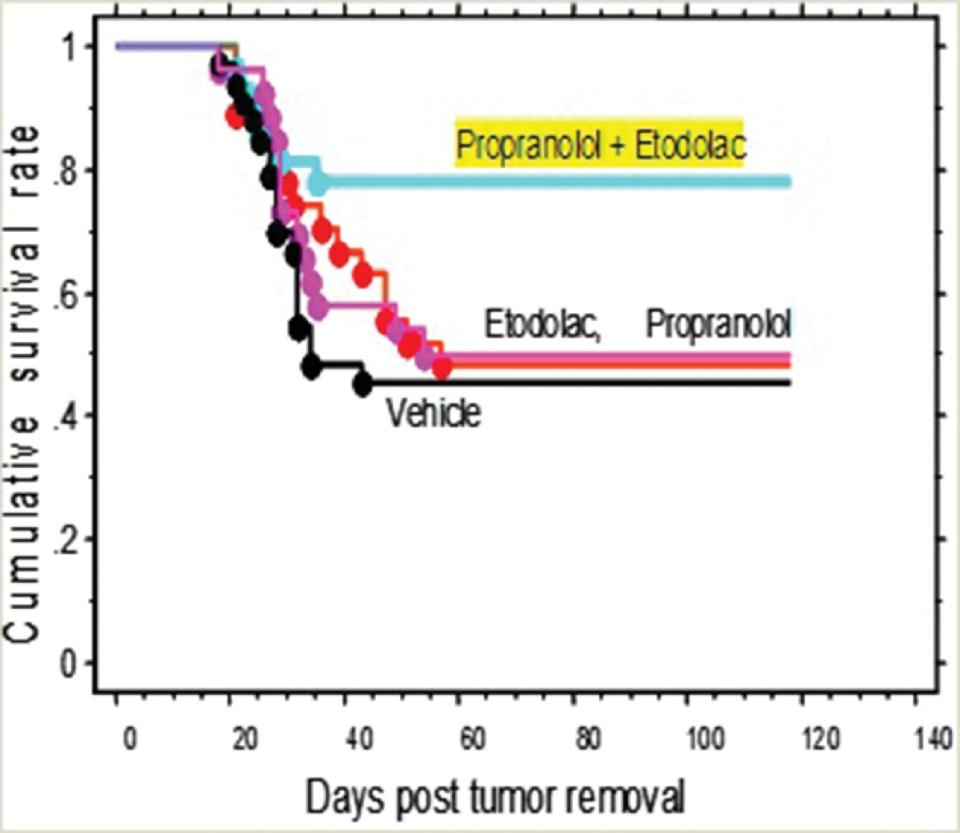
Based on preclinical studies, preventing the prometastatic effects of prostanoid and adrenergic signaling was suggested by us and others to be most effective in the initial stages of metastatic development. , , , , The perioperative period is characterized by high levels of CAs and PGs, and in most patients also by MRD in the form of scattered tumor cells and micrometastases. Several animal translational studies indicated that in the context of stress and/or surgery, the inhibition of inflammatory and/or adrenergic signaling can significantly reduce metastatic burden (e.g., number/weight of metastases) and increase survival rates , , , (see Figs. 9.2 and 9.3 for examples). As PGs and CAs converge to the same molecular pathways, in many animal models their combined blockade has been found to be particularly effective and in some models was the only effective approach using etodolac (semi-selective COX inhibitor) and propranolol (nonselective beta blocker). ,

Clinical studies regarding beta blockade and/or COX inhibition and their effects on cancer outcomes are mostly retrospective cohort studies and meta-analyses , , that have produced encouraging yet inconsistent evidence. , These studies, however, have only addressed the use of one family of drugs (i.e., beta-adrenergic blockers or COX inhibitors) used acutely or chronically, but not the combined effects of both agents. Drawing conclusions based on these studies is challenging as they are highly heterogeneous with respect to the type of medication, timing of treatment and treatment duration, cancer characteristics, heterogeneity of co morbid diseases, and selection bias of the chosen populations. Overall, the strongest retrospective evidence for perioperative or chronic use of COX inhibitors or beta-adrenergic blockers is reported in CRC and melanoma. , Importantly, NSAIDs are now recommended by government advisory boards as a first-line CRC prevention strategy, indicating their anticancer properties, as reflected in a recent recommendation by the “US preventive services task force” to men between the ages of 50–59 years. Furthermore, retrospective studies that have indicated beneficial effects of attenuating adrenergic or prostanoid signaling suggest that the use of a nonselective beta blocker (e.g., propranolol) may be more effective than selective blockers, and that the blockade of both COX1 and COX2 enzymes (e.g., using aspirin or etodolac) could be more advantageous than the blockade of each enzyme separately. , Taken together, robust evidence from animal studies and mixed results from retrospective human studies indicate potential benefits from blocking inflammation and/or adrenergic stress responses (ISRs) in the perioperative oncological context. As discussed later, recent small randomized controlled trials (RCTs) are now showing promising findings for the separate and/or combined perioperative use of beta blockers and NSAIDs.
Perioperative Beta-adrenergic Blockade in RCTs
Recent clinical trials provide evidence for the potential short- and possible long-term benefits of perioperative beta blockade. For example, in patients with ovarian cancer, three different RCTs have provided promising results. In one study, 5 days of perioperative oral propranolol (a nonselective beta blocker) treatment, initiated 2 days before surgery, reduced serum levels of CA-125 (a biomarker indicating cancer burden) for up to 3 weeks following surgery (n = 22). Another RCT studied the effects of low-dose (10–20 mg twice daily) propranolol, initiated 2 days prior to ovarian cancer surgery or as neoadjuvant therapy, and found decreased anxiety, depression, and increased quality of life (n = 32). A third RCT studying the effects of propranolol treatment (40 mg b.i.d.) initiated 3 days before surgery and continued until completion of chemotherapy treatment demonstrated significant reductions in VEGF, IL-6, MCP-1, and IL-8 serum levels at several time points along the treatment course (n = 84). These findings demonstrate the effectiveness of propranolol in reducing (i) markers of tumor burden, (ii) perioperative inflammation, and (iii) anxiety and distress.
A recent study assessing the effects of intraoperative intravenous landiolol hydrochloride (an ultra-short acting b1 blocker) administration to patients with lung cancer showed a trend toward increased 2-year relapse-free survival, with 89% (95% confidence interval [CI], 0.78–1.01) in landiolol-treated patients compared with 76% (95% CI, 0.6–0.91) in the placebo group, although this was not statistically significant ( P = 0.1828, n = 57). A recently concluded triple-blinded RCT study (n = 60) assessed the effects of an escalating dose of preoperative propranolol for 7 days prior to BC resection surgery. The trial focused on changes in prometastatic and proinflammatory gene expression between biopsy (prior to drug treatment) and tumor resection. Propranolol reduced mesenchymal gene expression ( P = 0.002), down-regulated intratumoral inflammatory transcription factors (Snail/Slug, NF-κB/Rel, AP-1), and promoted tumor infiltration of CD68 + macrophages and CD8 + T cells.
Taken together, clinical evidence from RCTs indicates the potential efficacy of perioperative beta blockade in reducing the metastatic potential of different cancers.
Perioperative RCTs of NSAIDs During Cancer Surgery
Evidence regarding the effects of perioperative NSAID use on cancer outcomes is also emerging. In a clinical trial of patients with thyroid cancer (n = 57), preoperative analgesia using intravenous parecoxib sodium (a highly selective COX2 inhibitor) reduced plasma levels of NE, cortisol, and blood glucose, indicating a reduced stress response to surgery through blockade of PG synthesis. Interestingly, this clinical evidence suggests that inhibition of COX2 may itself reduce local adrenergic (NE) signaling. In separate trials the use of perioperative parecoxib and flurbiprofen (nonselective COX inhibitor) enhanced antitumor immunity among patients with cervical and gastric cancer, restored Th1/Th2/Treg balance, and reduced postoperative suppression of CTLs and NK cell activity. In patients with CRC (n = 28), 3 days of preoperative NSAID treatment (the nonselective COX inhibitor indomethacin or the COX2 selective celecoxib) increased CTL infiltration into tumor stroma. Among patients with prostate cancer (n = 45), 4 weeks of presurgical treatment with celecoxib, initiated following biopsy, suggested that treatment decreased histological markers of proliferation and angiogenesis, and enhanced tumor apoptosis. Notably, standard dosing of celecoxib during cancer surgery only weakly reduced perioperative PG production, and post hoc analysis found that neo-CTX impaired celecoxib’s ability to reduce PGs. Thus it may be hypothesized that semi-selective COX inhibitors may be advantageous in some circumstances. In addition, effective reduction of perioperative inflammation may be assisted by beta blockade, either through intraoperative intravenous administration or oral administration initiated prior to surgery.
Perioperative Combined Blockade of Inflammatory Stress Responses
The rationale for clinical trials assessing the combined perioperative blockade of CA and PG signaling relies on the (i) copotentiating and synergistic pathways by which CAs and PGs affect cancer progression, (ii) simultaneous perioperative increase in both CAs and PGs, and (iii) preclinical studies indicating the superior impact of their combined blockade.
A recent retrospective study, published as an abstract, assessed the impact of perioperative separate and combined use of NSAIDs and/or beta blockers in patients with ovarian cancer, and found that their use was correlated with improved overall survival. Importantly, the combined use of propranolol and etodolac was correlated with a lower number of tumor nodules to a greater extent than each drug family used alone, suggesting that combined treatment increases inhibition of metastatic processes.
In two recently concluded RCTs, we treated BC , (n = 38) and CRC (n = 34) patients with a combination (see Tables 9.1 and 9.2 ) of etodolac and slow-release (SR) propranolol (vs. placebo). Treatment was initiated 5 days before surgery and continued for 5 (BC) or 14 days (CRC) following surgery. Etodolac was given at a dose of 400 mg b.i.d. throughout the entire intervention. SR propranolol was initiated at a dose of 20 mg b.i.d., increased to 80 mg b.i.d. on the day of surgery, and then down-titrated to 40 (only in CRC) and 20 mg postoperatively. Importantly, no drug-related adverse events were noted. In both CRC and BC tumor tissue samples extracted at surgery, 5 preoperative days of combined treatment led to decreased EMT and downregulation of proinflammatory and promalignant transcription pathways in the malignant tissue, including NF-κB, STAT-3, CREB, and the GATA family (see Fig. 9.4 ). The treatment also reduced monocyte presence in the tumor tissue; in BC patients the tumor proliferation marker Ki67 was reduced, and in CRC tumors NK cell infiltration was increased. Analyses of blood samples, which were collected only in the BC study, indicated that the perioperative treatment reduced serum IL-6 and CRP, improved markers of NK cell cytotoxicity, and enhanced IL-12- and IFN-γ-induced production, but did not affect antiinflammatory IL-10 and cortisol serum levels. Finally, the increase in circulating monocyte number on postoperative day 1 was completely abolished by the treatment. Notably, in the CRC study, the 3 years following surgery recurrence rates were 1/15 in treated patients, and 5/19 in the placebo group (see Fig. 9.5 ) ( P = 0.23) in intent to treat analysis, and 0/11 vs. 5/17 in protocol compliant patients ( P = 0.054). Taken together, recent studies in the clinical setting indicate that the combined approach improves perioperative immune function and reduces prometastatic processes. Efforts to examine the long-term significance of these results are now underway in two RCTs in CRC (NCT03919461) and pancreatic (NCT03838029) cancer patients.









