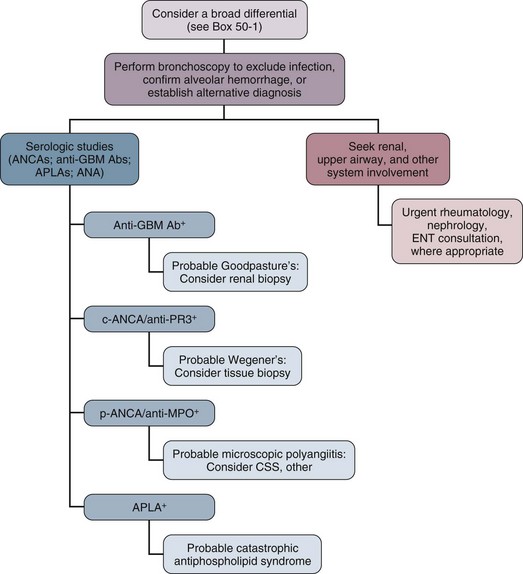
49 The lungs of patients with immunologic diseases, in particular, interstitial lung diseases such as IPF and connective tissue lung diseases, exhibit restrictive physiology with decreased lung volumes, decreased parenchymal compliance, and a loss of functional capillary beds leading to a reduction in the diffusing capacity.1 In this manner the functionally smaller lungs (baby lungs) of these patients resemble the lungs of patients with the acute respiratory distress syndrome (ARDS). This concept is supported by computed tomography (CT) findings of heterogeneous disease involvement in the lungs of patients with both IPF and ARDS. This parallel can be used as a framework for managing tidal volumes during mechanical ventilation of patients with immunologic lung disease and respiratory failure. If large tidal volumes (or excessive inflation pressures) are used, relatively normal areas of lung will be overdistended, potentially exacerbating lung injury. Limiting tidal volumes to 6 mL/kg predicted body weight in patients with acute lung injury (ALI) or ARDS saves lives.2 Similar data are not available regarding safe parameters for ventilating patients with chronic restrictive lung diseases, but limiting tidal volumes to roughly 6 mL/kg (and raising the rate accordingly) carries little risk. Moreover, retrospective analysis of mechanically ventilated patients without ALI/ARDS suggests that large tidal volumes may produce ALI (odds ratio 1.3 for each milliliter above 6 mL/kg predicted body weight).3 Because there is little evidence that intrinsic positive end-expiratory pressure (PEEP) plays a physiologically important role in respiratory failure in patients with interstitial lung disease, rapid ventilatory rates are tolerated.1 This approach may allow ventilation without resorting to permissive hypercapnia, which may aggravate pulmonary hypertension.4,5 As in the ARDS lung, it seems likely that nonfunctional, diseased lung units exist alongside those with essentially normal function. However, unlike the acutely injured lung, atelectatic lung units available for recruitment through the use of elevated end-expiratory pressure are rare in conditions such as IPF. There is probably little clinical advantage to using high PEEP in patients with restrictive lung diseases; in fact, high levels of PEEP may be detrimental by overdistending the lung, as well as by contributing to cor pulmonale, as described later. In a cohort of ventilated patients with interstitial lung disease, high PEEP during the first 24 hours of mechanical ventilation was one of the independent determinants of death.6 Although it is likely that high PEEP served as a marker (rather than a cause) of severity in this study, high PEEP should nevertheless be applied cautiously to minimize harm. Another clinicopathologic process to consider in the ventilatory management of the patient with immunologic lung disease is pulmonary hypertension. Pulmonary artery pressures are chronically elevated in advanced stages with lung fibrosis, and this pressure increases further with the increased cardiac output that accompanies exercise, fever, and hypercarbia.5,7 Pulmonary hypertension may eventually lead to cor pulmonale because of increased right ventricular afterload.8 Mechanical ventilation may interact adversely with pulmonary hypertension. Positive-pressure ventilation alone impairs right-sided heart function, and this effect is exaggerated by PEEP.9 PEEP increases afterload by increasing pulmonary vascular resistance.10 The resulting increase in wall tension decreases right ventricular perfusion, which leads to myocardial ischemia.10,11 Right ventricular ischemia may cause further dysfunction and dilation of the right side of the heart, in addition to diastolic dysfunction of the left ventricle (through ventricular interdependence), producing a cycle of progressively deteriorating circulatory function.8 Thus, it is important to minimize further increases in pulmonary artery pressure and to maintain adequate systemic pressure to preserve perfusion of the right ventricle. Additionally, adequate oxygenation is essential to prevent reflex increases in pulmonary artery pressure and to maintain peripheral oxygen delivery. Finally, hypercapnia, which tends to raise pulmonary artery pressures, should generally be avoided. Because predicting the degree of pulmonary hypertension clinically is difficult—and the need to avoid increases in pulmonary artery pressures is so important—we advocate liberal use of echocardiography. Other forms of monitoring, such as central venous saturation measurement or pulmonary artery catheterization, might also be useful. When acute-on-chronic cor pulmonale compromises the circulation, dobutamine or norepinephrine is often helpful.12 Inhaled nitric oxide or inhaled prostacyclin probably plays some role, at least to buy time in the critically impaired patient.13,14 The role of newer pulmonary vasodilators such as bosentan and sildenafil in patients with acute cor pulmonale is unclear. Rescue therapies such as extracorporeal membrane oxygenation or pumpless extracorporeal lung assist devices may be of benefit in selected cases when used early.15,16 Data on noninvasive mechanical ventilation in immunologic lung disease is limited. Two small studies show that noninvasive ventilation can be used to avoid intubation and risk for ventilator-associated pneumonia with comparable or better short-term survival.17,18 Idiopathic pulmonary fibrosis is a disorder of unknown cause characterized by inflammation of the lower respiratory tract that usually leads to irreversible scarring. Current and former smokers are at increased risk, and there may be an inherited susceptibility to develop this disease.19–21 IPF most commonly presents as an outpatient illness with the insidious onset of exertional dyspnea and cough. On examination of the lungs, coarse crackles are found and clubbing of the fingers is characteristic.21 Chest radiographs may show a spectrum of findings from peripheral reticular densities to end-stage honeycombed lung.21 Alveolar infiltrates are unusual unless the patient has a concurrent lung cancer, pneumonia, or heart disease. The lung CT findings most closely associated with a pathologic diagnosis of IPF are lower-lung honeycombing and upper-lung irregular lines.22 The histologic examination of IPF reveals usual interstitial pneumonitis, which is characterized by inflammation, fibroblastic foci, areas of fibrosis, and remodeling of the lung parenchyma. The pulmonary fibrosis appears to follow collapse of involved alveoli. Death in patients with IPF is most often directly attributable to progression of the underlying disease, even when the disease is only of moderate severity.21,23 Nevertheless, the clinician should seek treatable complicating conditions before making the difficult decision to withhold mechanical ventilatory support. Other causes of respiratory failure in IPF include infection, congestive heart failure, bronchogenic carcinoma, pulmonary embolism, and pneumothorax. Left ventricular failure is often found in association with IPF. These patients often have many of the risk factors (e.g., smoking, hyperlipidemia) that are associated with the development of atherosclerosis. For this reason, left ventricular failure may result from ischemic heart disease. Two other factors that may contribute to left ventricular failure in these individuals are systemic arterial hypertension and right ventricular failure. Hypoxemia may exacerbate these effects. A search for potentially treatable left ventricular failure should be considered in the deteriorating IPF patient. Patients with IPF have about a 14-fold excess risk of developing lung cancer.24 These malignancies are difficult to detect on an already abnormal chest radiograph and often cause rapid deterioration in the IPF patient. Treatment options for malignancy are often limited by poor pulmonary reserve. However, the diagnosis of lung cancer may greatly alter therapeutic planning. Furthermore, relieving airway obstruction and postobstructive infection may significantly palliate dyspnea. Pulmonary embolism can also cause rapid deterioration and respiratory failure in IPF patients. Ventilation/perfusion scans often reveal nonsegmental perfusion defects and inhomogeneous areas of poor ventilation as a result of the IPF alone, so the utility of these scans in diagnosing pulmonary emboli is limited.25 Pulmonary angiography or helical CT scanning should be considered if it can be performed safely.25 We recommend empiric long-term anticoagulation for individuals with advanced IPF and severe pulmonary hypertension who are suspected of having pulmonary embolism but for whom a diagnostic evaluation is not feasible.26 An important factor to consider in patients with advanced IPF who develop respiratory failure is that this disease is largely irreversible. Although many exciting new therapies are under investigation, current treatments are largely ineffective in reversing the decline in lung function.27–29 Most patients with IPF who are in respiratory failure do not respond to corticosteroid therapy.30 Cytotoxic therapy, such as cyclophosphamide, azathioprine, or cyclosporine, has not been shown to alter survival.31,32 Mortality rate after ICU admission is high, raising the question of appropriateness of mechanical ventilation in most cases, with the exception of perioperative support or as a bridge to lung transplantation.31,33–35 In a review of nine studies examining 135 patients with IPF ventilated in the ICU, the aggregate hospital mortality rate was 87% and the mortality rate within 3 months after discharge was 94%.36 If patients are young, have early disease, or may be diagnosed with interstitial lung disease other than IPF, an open lung biopsy should be considered to exclude alternative treatable diseases. Recently, lung transplantation has become a viable option for some patients with end-stage IPF. It is imperative that physicians caring for these patients familiarize themselves with the referral protocols and policies of their respective regional transplant centers. Hamman-Rich syndrome, more recently called acute interstitial pneumonia (AIP), is a rapidly progressive interstitial pneumonia of unknown cause first described by Hamman and Rich.37 The mean age of patients is 50 to 60 years, with a broad range and perhaps an increased risk for men.37,38 The patients often describe a prodromal viral-like respiratory illness typically followed by subacute progressive dyspnea, fever, and nonproductive cough. AIP usually evolves over 1 to 3 months, and in some instances, it appears within 1 to 2 weeks after the onset of symptoms. Signs of right-sided heart failure may exist, and diffuse or basilar crackles may be found on auscultation of the lung. Diffuse, bilateral interstitial infiltrates are characteristic on chest radiograph. The findings on CT scan include diffuse, patchy alveolar ground glass infiltrates and pleural effusion in one third of the cases.39 Honeycombing may be present in subacute cases. Laboratory studies may show a leukocytosis with neutrophilia. Hypoxemia may be profound. Pulmonary function tests in patients without respiratory failure show a restrictive defect, generally without evidence of airway obstruction.38 For many years experts believed that this disease was simply a rapidly progressive form of IPF; now this disease is felt to be more related to ARDS. The pathologic features of AIP are characterized by diffuse, active fibrosis, with proliferating fibroblasts and minimal collagen. These findings appear acute and relatively uniform in age and resemble the organizing stage of diffuse alveolar damage as seen in ARDS.40 The prognosis of acute interstitial pneumonitis is poor, with only about a 40% short-term survival rate. Although the short-term mortality rate is similar to that for acute exacerbation of IPF, AIP survivors have near complete recovery of lung function in contrast to those with IPF.41,42 Supportive care may involve ventilatory support. Antibiotics to treat possible underlying infection and corticosteroids to treat inflammation have been used in many cases, but the efficacy of these treatments is not proved.38 In one small series, early efforts to exclude infection combined with lung-protective ventilation and high-dose corticosteroid therapy led to success in 8 of 10 patients.43 We recommend rigorous exclusion of an infectious cause including open lung biopsy, if necessary, before considering any immunosuppressive therapy. Perhaps the most striking immunologically mediated lung diseases are those that present with alveolar hemorrhage. These disorders require prompt diagnosis and management. We limit our comments here to the disorders that most commonly present as alveolar hemorrhage: Good-pasture’s syndrome, Wegener’s granulomatosis (WG), microscopic polyangiitis (MPA), catastrophic antiphospholipid syndrome (CAPS), systemic lupus erythematosus (SLE), and idiopathic pulmonary hemosiderosis. Box 49.1 provides a more complete list of disorders that can lead to alveolar hemorrhage. An essential goal of managing patients with alveolar hemorrhage is prompt diagnosis of the underlying disorder (Fig. 49.1). The first step is to document alveolar hemorrhage. The classic triad of hemoptysis, anemia, and diffuse infiltrates on chest radiograph strongly suggests alveolar hemorrhage, yet many patients with significant alveolar hemorrhage do not have hemoptysis.44 Consequently, the absence of hemoptysis does not exclude the presence of alveolar hemorrhage. Thus, diffuse pulmonary infiltrates, respiratory distress, and anemia associated with clinical evidence of glomerulonephritis or other conditions associated with vasculitis should arouse suspicion for alveolar hemorrhage, even in the absence of hemoptysis. Before any specific therapy is instituted, it is important to document the presence of alveolar hemorrhage. Other processes that result in diffuse alveolar filling must be excluded, such as inflammatory exudate from infection, cardiogenic pulmonary edema, and ARDS. In addition, one should exclude hemorrhage from airway sources such as cancer, bronchitis, bronchiectasis, or excessive anticoagulation or an endogenous coagulation defect. Perhaps the most valuable test for documenting alveolar hemorrhage is bronchoscopy with BAL. Blood-tinged lavage fluid or frank blood in the airways is usually present.45 Another test that can be used involves staining alveolar macrophages retrieved by lavage for hemosiderin. Normal individuals will have few hemosiderin-laden macrophages in BAL, but the intensity of staining and percentage of cells staining positive have been found to be predictive of alveolar hemorrhage.46,47 We feel, however, that this test is of questionable clinical value. If patients have sufficient acute bleeding to cause infiltrates on chest radiograph, this should be seen easily in the lavage fluid. If the only evidence for hemorrhage is the presence of hemosiderin-laden macrophages, we would propose that the acute infiltrates are the result of another cause. Similarly, the negative predictive value of this test can be questioned because it may take up to 48 hours for intracellular hemosiderin accumulation after an acute bleed.48 Documentation of an elevated diffusion capacity for carbon monoxide (DLCO) is also a means of evaluating for alveolar hemorrhage but is not practical during active bleeding or critical illness.49,50 Bowley and coworkers demonstrated the usefulness of this measure as a sensitive index of recurrent alveolar hemorrhage in patients undergoing treatment.50 Treatment consists of supportive care including mechanical ventilation, prevention of infections and organ damage and immunosuppressive therapy directed at the underlying process. Rescue therapies such as recombinant factor VIIa and extracorporeal membrane oxygenation have been reported to be successful in anecdotal cases of refractory alveolar hemorrhage.51–55 Survival following alveolar hemorrhage due to immunologic lung disease tends to be better compared to patients with alveolar hemorrhage from thrombocytopenia or sepsis.56 Goodpasture’s syndrome accounts for 20% to 30% of the cases of alveolar hemorrhage.57 This disease is a classic pulmonary-renal syndrome with a high mortality rate from alveolar hemorrhage or renal failure if untreated. Anti–basement membrane antibody is a universal finding in this disease. Antibody deposition along the glomerular basement membrane (GBM) undoubtedly contributes to the renal pathologic examination of this disease; however, other cofactors, in addition to anti-GBM antibody, may be necessary for alveolar hemorrhage to develop. A higher incidence of alveolar hemorrhage has been reported in smokers with anti-GBM antibody disease.58 Experimental studies also showed that exposure to 100% oxygen in animals with circulating anti–basement membrane antibody resulted in alveolar hemorrhage, whereas unexposed animals did not develop lung disease.59 Genetic studies have shown a strong association with HLA-DRB alleles.60,61 A 2:1 male-female ratio exists with a median age of 21 years in patients with Goodpasture’s syndrome.62,63 Alveolar hemorrhage is the most common presentation of Goodpasture’s syndrome. Evidence of renal involvement is usually present; however, some patients may only have microscopic hematuria.57 Untreated, Goodpasture’s syndrome carries a mortality rate approaching 100%, the cause of death being equally divided between uremia and alveolar hemorrhage.63 With prompt dialysis, plasma exchange, and immunosuppression, however, the acute mortality rate of the disease is about 10%, with diffuse alveolar hemorrhage being the most common cause of early death.64 The evaluation of patients suspected of having Goodpasture’s syndrome should include confirmation of alveolar hemorrhage, evaluation for renal disease, and testing for anti–basement membrane antibody. Circulating anti–basement membrane antibody can be demonstrated in more than 95% of patients through radioimmunoassay or enzyme-linked immunosorbent assay (ELISA).65 Kidney biopsy should also be considered to confirm the diagnosis and to document the extent of glomerular loss. The characteristic glomerular lesion shows strong linear deposition of immunoglobulin G (IgG) along glomerular capillaries.57 Other histologic features include segmental, necrotizing, crescentic glomerulonephritis indistinguishable from that found in other forms of vasculitis. Walker and colleagues66 demonstrated that patients with greater than 85% crescents on biopsy were significantly less likely to regain renal function. Lung biopsy is rarely necessary and often nonspecific. Treatment consists of dialysis, plasma exchange, and immunosuppressive therapy. Immunosuppression usually includes both cyclophosphamide and corticosteroids.67 Dialysis should be performed early to reverse uremic platelet dysfunction and to prevent fluid overload because both factors may perpetuate alveolar hemorrhage. Mechanical ventilation may be necessary to provide respiratory support, as well as to facilitate clearing blood from the airways. When mechanical ventilatory support is used, efforts should be made to select lung-protective tidal volumes and to minimize the fraction of inspired oxygen. We also aggressively treat possible respiratory infection because it may precipitate and perpetuate alveolar hemorrhage. If the patient has received drugs that impair platelet function, such as aspirin, we also administer platelets in cases of life-threatening hemorrhage. Alveolar hemorrhage generally responds within 1 to 3 days to this treatment.68 In refractory cases, there has been anecdotal response to mycophenolate or to anti-CD20 antibody. Once the patient has recovered, the importance of maintenance immunosuppression in preventing recurrent alveolar hemorrhage cannot be overemphasized.57,68,69 Another form of vasculitis that commonly presents as alveolar hemorrhage is WG. This disorder is characterized by a granulomatous vasculitis involving the upper and lower airways and is associated with rapidly progressive renal failure. WG represented 15% of the cases of alveolar hemorrhage in a series reported by Leatherman.57 The incidence of pulmonary hemorrhage in this disorder is reported to vary between 12% and 30%.70,71 Clinical findings that may suggest a diagnosis of WG include nodules visible on a chest radiograph and evidence of upper airway involvement including chronic otitis media, sinusitis, nasal septal perforation, and tracheal stenosis.72 Eye involvement with either proptosis or extraocular muscle entrapment may occur.72 Skin lesions may include petechiae, palpable purpura, ulcers, vesicles, papules, and subcutaneous nodules.70 Musculoskeletal findings include myalgias, arthralgias, and pauciarticular or migratory arthritis. Neurologic manifestations include sensorineural deafness, mononeuritis multiplex, and cranial nerve palsies.70,72 Subglottic tracheal stenosis or obstruction can occur in these patients and should be considered before endotracheal intubation is undertaken. In up to 10% of patients, tracheostomy may be required to manage the airway at some time during the course of their illness.72 Laboratory findings in WG include leukocytosis, anemia, thrombocytosis, and an elevated erythrocyte sedimentation rate. A valuable laboratory test is the antineutrophil cytoplasmic antibody (ANCA), which detects IgG directed at a variety of neutrophil and monocyte antigens. Clinically important ANCAs are of two types: antiproteinase 3 antibodies (anti-PR3) and antimyeloperoxidase antibodies (anti-MPO). When serum containing these antibodies is applied to neutrophils and stained by indirect immunofluorescence, anti-PR3 produces a cytoplasmic pattern of staining (c-ANCA), whereas anti-MPO produces a perinuclear or nuclear pattern (p-ANCA). Both anti-PR3 (seen almost exclusively in WG) and anti-MPO (which may be seen in pauci-immune rapidly progressive glomerulonephritis, Churg-Strauss syndrome, and MPA) can be measured more directly by ELISA. The sensitivity of ANCA has been reported to be 80% to 96% in patients with active generalized (e.g., having renal involvement) WG, and generally these patients have both c-ANCA and anti-PR3 positivity. In the ANCA-associated systemic vasculitides, which include WG, alveolar hemorrhage has been found in patients who have tested positive for either c-ANCA or p-ANCA (see “Microscopic Polyangiitis” later). More recently, the presence of an immunoglobulin M (IgM) isotype of ANCA has been strongly associated with alveolar hemorrhage; conversely, patients lacking IgM ANCA may have a low risk for alveolar hemorrhage.73 Frequently the diagnosis of WG relies on tissue examination. Biopsies of upper airway lesions are probably acceptable in nonemergent situations when diagnosis can be delayed. However, in the case of severe alveolar hemorrhage, there is frequently an emergent need for diagnosis so that effective treatment can be instituted. An open lung biopsy often provides the diagnosis. Potential infectious causes, especially mycobacterial and fungal pathogens, can also be excluded. Fauci and coworkers have developed a scheme of major and minor criteria for WG on the basis of histologic diagnosis. Three major pathologic manifestations were identified including parenchymal necrosis, vasculitis, and granulomatous inflammation74; however, in 18% of biopsies, less distinctive histologic features were the predominant findings. If definitive tissue biopsy cannot be obtained, a WG with alveolar hemorrhage diagnosis should be based on the histologic finding of a small vessel vasculitis or crescentic glomerulonephritis, together with compelling clinical evidence of WG consisting of cavitary pulmonary nodules or characteristic upper airway involvement.57 Moreover, the presence of a positive c-ANCA test can help to confirm the diagnosis.
Immunologic Lung Disease in the Critically Ill
Clinicopathologic Considerations during Mechanical Ventilation
Idiopathic Pulmonary Fibrosis
Hamman-Rich Syndrome
Alveolar Hemorrhage Syndromes
Goodpasture’s Syndrome
Wegener’s Granulomatosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Immunologic Lung Disease in the Critically Ill