Fig. 74.1
Chest CT. A CT of the chest show a right sided cavitary lesion concerning for fungal pneumonia
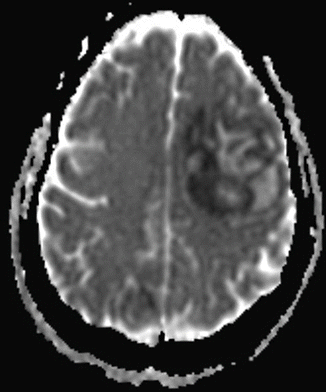
Fig. 74.2
Head CT. A CT of the head reveals a mass lesion concerning for spread of her fungal infection
Question
How does time from transplant affect consideration of which complications may be present?
Answer
Complications following HCT pertain to the patient’s immune statuse and may be roughly divided into three “phases:” pre-engraftment during approximately the first month; engraftment during months 2-3; and post-engraftment after approximately the first 100 days following HCT.
Due to the transplant and the therapies an HCT patient receives for maintenance, the innate and acquired immune systems of this patient population do not function normally. This places these patients at increased risk for a host of infectious and non-infectious diseases.
Common ICU Admission Etiologies in HCT Patient
Respiratory Failure
See Etiologies of Respiratory Failure on HCT Patient below
Shock
Septic shock
Hypovolemic shock
Cardiogenic shock
Obstructive shock (thrombosis)
Renal failure resulting in metabolic derangements
Hyperkalemia
Acidosis
Gastrointestinal System
GI bleeding
Enterocolitis
Liver failure
Central Nervous system
Intracranial hemorrhage
Seizure
Altered Mental Status
Currently there are greater than 50,000 HCTs performed yearly [1] thus, there are many patients at all stages in the post-transplant periods that could potentially develop a process requiring admission the ICU. This patient experienced several devastating complications related to her HCT including graft-versus-host disease, respiratory failure, and opportunistic infection. This chapter will focus on these issues that are commonplace in HCT patients that are admitted to the ICU, in particular, the impact of GVHD and the pulmonary complications of HCT.
Principles of Management
Hematopoietic stem cell transplantation has become a relatively common procedure worldwide with a diverse array of indications. The indications range from leukemias and lymphomas to inborn errors of metabolism to autoimmune diseases. Other considerations include patient age, functional status, and suitable graft availability. There are four sources for a hematopoietic stem cell transplant: autologous transplantation refers to a patient receiving his own stem cells, in syngeneic transplantation a patient receives stem cells from an identical twin sibling, in allogeneic transplantation a patient receives stem cells from a non-identical sibling or an unrelated donor and in umbilical cord blood transplantation stem cells harvested from the umbilical cord and placental soon after a baby is born. In allogeneic transplantation, recipient and donor human leukocyte-associated antigens (HLA) are matched as best as possible. Graft-versus-host disease is common in allogeneic transplants and uncommon in autologous transplants. GVHD is generally more severe in the patients receiving transplants from mismatched donors [2].
In preparation for the transplant, conditioning regimens are used to treat the patient prior to the transplant and the severity of graft-versus-host disease appears be influenced by the conditioning regimen chosen [3]. The regimens are meant to suppress the recipient’s immune system enough that the graft is not rejected as well as eliminate the disease for which the patient is receiving the transplant. The myeloablative regimens are meant to completely destroy the recipient’s stem cell population and regimens often are made up of both chemotherapy and total body irradiation (TBI). Non-myeloablative regimens generally use lower doses of chemotherapy and TBI and are not designed to completely eradicate the recipient’s stem cell (or diseased cell) population. Eradication of the malignancy relies on the graft-versus tumor effect provided by the donor stem cells. Choice of regimen is influenced by the patient’s co-morbities, the condition that is being treated, the status of the disease being treated, and the likelihood of graft rejection [2].
Impact of Graft-Versus-Host Disease on the Critically Ill HCT Patient
Importantly, recipients of allogeneic HCT commonly develop GVHD and the graft versus tumor effect is generally associated with reduced relapse of malignancy however GVHD (both acute and chronic) is associated with significant morbidity and mortality [4]. GVHD is a common multisystem “side-effect” of HCT seen in patients with allogeneic HCT. It occurs when donor hematopoietic cells recognize the recipient’s organ tissues as foreign resulting in end-organ injury [5]. Acute GVHD typically occurs within 100 days of transplantation and is characterized by skin, GI tract and liver involvement. However, all organ systems can be involved [6]. Chronic GVHD commonly occurs outside the first 100 days after HCT though the clinical manifestations noted in the patient as opposed to the timing are perhaps more important in making the diagnosis of acute or chronic GVHD. While skin, GI tract and liver are also commonly involved in chronic GVHD, the findings of chronic GVHD are much different than what is seen in acute GVHD and tend to have an appearance similar to that of a patient with advanced autoimmune disease. Sclerotic skin lesions, which are strikingly similar to the cutaneous findings of scleroderma, are commonplace in chronic GVHD [7]. Both acute and chronic GVHD are associated with significant non-relapse morbidity and mortality in the HCT patient [8].
Mortality after HCT is higher in patients who have GVHD as opposed to those who do not and as the severity of GVHD increases, so does mortality. It has also been demonstrated that patients who receive allogeneic transplants from unrelated donors also have higher rates of GVHD and non-relapse mortality [9]. Al-Khadhimi et al. retrospectively evaluated 414 patients with related and unrelated HCT and demonstrated the incidence of severe acute GVHD was much higher in the recipient from unrelated donors (22.3 % vs 36.5 %). It was also shown that non-relapse mortality was significantly higher (33.3 % vs 46.5 %) at 60 months. In this cohort, only 15 % of patients with the severest (grade III and IV) level of acute GVHD were alive at 5 years [10]. While the clinical manifestations of acute and chronic GVHD share some similarities, having a history of acute GVHD is, in and of itself, a risk factor for chronic GVHD [11].
The patient described developed multiple manifestations of chronic GVHD disease including skin, ocular, and pulmonary. Bronchiolitis obliterans syndrome (BOS) is a common pulmonary manifestation of chronic GVHD and is thought to occur in between 1.7 and 26 % of patient receiving allogeneic HCT [12]. In a study by Au et al., the survival analysis hazard ratio was 1.6 for HCT patients with BOS when compared with those with HCT without BOS. Its incidence is closely associated with chronic GVHD, prior history of acute GVHD, older donor and recipient age, poor lung function prior to transplantation, early post-transplant respiratory viral infections, and the type of conditioning regimen prior to transplant [12]. Treatment generally includes systemic corticosteroids with and without other immune suppressing medications. The immune suppressing regimen often includes a prolonged course of high dose prednisone and in the event there is minimal response to prednisone, calcineurin inhibitors are added [13, 14]. Unfortunately, BOS often progresses despite attempts at therapy. These treatments for BOS often result in medication side effects and increased risk of opportunistic infections due to their significant immune suppressing properties [14].
Pulmonary Complications in the GVHD Patient
Respiratory failure, as occurred in this patient, exemplifies a common, yet heterogeneous problem in any ICU population. However, the HCT population is a unique group in which certain infectious and non-infectious pulmonary complications can be expected at various periods post-HCT. A retrospective review of 250 consecutive HCT patients, of who 33 were admitted to the ICU and the most common reason for ICU admit were pulmonary complications [16]. Indeed, respiratory failure carries a particularly poor prognosis in this population and can be a result of both infectious and non-infectious etiologies.
Etiologies of Respiratory Failure in HCT Patient
Infectious
Bacteria:
GPC, GNR, polymicrobial with aspiration
Viruses:
CMV, Herpesviruses, Respiratory viruses
Fungus:
Aspergillus, Candida, Mucormycoses
Mycobacteria:
Tuberculosis, non-tuberculous mycobacteria
Non–Infectious
Diffuse Alveolar Hemorrhage
Idiopathic Pneumonia syndrome
Cryptogenic Organizing Pneumonia
Pulmonary Edema
Bronchiolitis Obliterans Syndrome
Pulmonary veno-occlusive disease
Radiation Pneumonitis
Due to the immunologic derangements and subsequent immune system recovery that occurs after an HCT, the patient will be at higher risk for numerous pulmonary complications depending on the post-transplant phase.
Recovery “Phases” Post-HCT
Recovery from HCT can divided into three phases which is helpful in allowing the physician rapidly assess and treat a broad differential. Phase 1 is the period immediately post-transplant to the point of cellular engraftment (defined as an absolute neutrophil count >0.5 × 109 cells/L), which occurs about 3–4 weeks post-transplant. Many of the infectious pulmonary complications in this period are related to the neutropenia that make risk of opportunistic infection from bacteria, fungi, and viruses commonplace. Phase 2 encompasses the period from engraftment to the point at which full immunity from the allograft is conferred, which can take up to 100 days post-transplant. During this period, humoral and cell-mediated immunity remain impaired. The final recovery stage, phase 3, commonly is characterized by ongoing defects in cell-mediated and humoral immunity, as well as diminished function of the reticuloendothelial system.
Common Infectious Complications of Phase 1
Bacterial infections, including gram negative, gram positive and anaerobic organisms, are commonplace during phase 1 due to the resultant neutropenia from the regimens used to perform the HCT itself. Patients who experience mucositis during phase 1 are at increased risk of bacterial translocation into the bloodstream and also aspiration from the dysphagia they often experience which can result in bacterial pneumonias. As such, during phase 1, when patients present with fever they require empiric broad spectrum antibiotic prophylaxis to treat both gram positive and gram negative organisms even if the source of the fever is elusive [17].
During the prolonged periods of neutropenia of phase 1, opportunistic infections from invasive Aspergillus are commonly seen, even with anti-fungal prophylaxis [18]. Notable risk factors include allogeneic HCT, acute GVHD, and older age. Clinical features often include dyspnea, fever, pleuritic chest pain and occasionally hemoptysis. Imaging will often show nodular opacities on chest radiograph and the “halo” sign on CT scanning. Treatment is generally with voriconazole or amphotericin, though the echinocandins are also used [19].
Herpesviruses are common viral infections seen in phase 1 [20, 21]. Disease activity results from reactivation of latent infection. In patients who do not receive prophylaxis with acyclovir, HSV will be shed in up to 80 % of seropositive patients. HSV pneumonitis requires evidence of tissue invasion for diagnosis and generally patients with pneumonitis will also have evidence of mucocutaneous involvement. Imaging can demonstrate diffuse or patchy opacities [21]. Treatment is generally with acyclovir.
Common Non-Infectious Phase 1 Pulmonary Complications of HCT
Diffuse alveolar hemorrhage (DAH), a phenomena occurring more commonly in autologous HCT than allogeneic HCTs, is characterized by fever, hypoxemia, coughing, and central bilateral pulmonary opacities. The diagnosis of DAH is made on bronchoalveolar lavage (BAL) when non-clearing hemorrhagic return is seen on successive lavage aliquots [22]. Due to the patchy nature of DAH, performing BALs in multiple areas of the lung may be required to establish a diagnosis. As with other non-infectious complications of HCT, there must be no evidence of infection present to establish a diagnosis of DAH. Corticosteroids are often used for treatment, though the in-hospital mortality of DAH approaches 80 % [23].
During phase 1, patients regularly develop pulmonary edema which can be either cardiogenic or non-cardiogenic in nature [24]. It is characterized by dyspnea, hypoxemia, bilateral rales, bilateral pulmonary interstitial opacities, and occasionally associated with pleural effusion. The etiology of the pulmonary edema is diverse though patients often have cardiac or renal dysfunction as a result of previous chemotherapeutics used (platinum based, anthracyclines, or cyclophosphamide being the most common), and hypoalbuminemia can also play a role. Increased vascular permeability as a result of sepsis, viral infection, aspiration, or hyper acute GVHD can result in non-cardiogenic pulmonary edema [25]. Typically, pulmonary edema will improve with diuresis and often has a good prognosis though patients with hepatic veno-occlusive disease can develop pulmonary edema that has significantly worse outcomes [26].
Engraftment syndrome occurs in about 7–10 % of patients with autologous HCT and is less common in patients with allogeneic HCT. Common features include fever, dyspnea, hypoxia, maculopapular rash (not attributable to medication), and bilateral pulmonary opacities. This syndrome occurs at the same time as the patient’s neutrophils recover. Infectious and cardiogenic etiologies must be ruled out to make the diagnosis. Patients tend to have a better prognosis than many of the other etiologies of respiratory failure and one case series reported excellent resolution of hypoxia and fever when treated with corticosteroids [27].
Common Infectious and Non-Infectious Pulmonary Complications in Phase 2
In the immunocompetent host, Cytomegalovirus (CMV) remains latent in the host’s leukocytes though will reactivate in the seropositive patient who becomes immunosuppressed. Most HCT patient receive prophylaxis for CMV and as such, CMV pneumonitis is no longer as common as previously in the transplant population [28]. It is seen in about 5 % of HCT patients and tends to occur between 6 and 12 weeks post-transplant. As is commonly seen with many forms of pneumonitis, it is characterized by fevers, dyspnea, hypoxia, and diffuse opacities on radiograph. Primary risk factors for CMV include previous CMV viremia, GVHD and use of high-dose glucocorticoids. The treatment typically includes intra-venous (IV) ganciclovir or valganciclovir. The mortality in the HCT patient with CMV pneumonia is quite high at 80–90 % [29].
There are a number of other viruses known to cause pulmonary complications in the HCT patient including RSV, influenza, parainfluenza, and human metapneumonvirus. While some may not carry the high mortality associated with CMV pneumonitis, most carry significant morbidity and mortality when they result in pneumonia [30].
The idiopathic pneumonia syndrome (IPS) is a common non-infectious complication of phase 2. This usually occurs 4 months post-HCT and is characterized by two phases: an early inflammatory phase followed by a fibrotic phase likely resulting from an impaired ability to recover in a normal fashion from an insult leading to injury [31]. The diagnosis of IPS is predicated on the fulfillment of two criteria: diffuse alveolar injury with symptoms suggestive of pneumonia without evidence of congestive heart failure or volume overload and no evidence of lower respiratory tract infection. Risk factors for IPS include the diagnosis of acute leukemia, the type of conditioning regimen – myleoablative, total body irradiation, severe acute GVHD, and age greater than 40 [31, 32]. A compelling study published in Blood in 2015 re-evaluated BAL samples of 69 patients previously diagnosed with IPS between 1992 and 2006 using PCR screening for 3 bacteria and 25 viruses. Pathogens were discovered in 39 patients, a significant proportion of whom had worse 100 day survival than those without pathogens. While the significance of these findings are uncertain, they suggest that aggressive search for infectious pathogens must be undertaken prior to making the diagnosis of IPS [33]. The HCT patient diagnosed with IPS is typically treated with high-dose corticosteroids though, unfortunately, prognosis is quite poor and ranges from 50 to 75 % [34]. More recently, a study looking at the etanercept in addition to corticosteroids did not confer an improved response. Interestingly, this study demonstrated better 28-day survival rates than historically documented perhaps owing to the fact that conditioning regimens, supportive care and frequency of BAL (thus allowing for identification of pathogens) has changed [35]. Unfortunately the 1-year survival rates post-IPS are high [34].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







