INTRODUCTION
Hydrocarbons are a diverse group of organic compounds consisting primarily of carbon and hydrogen atoms. The two basic forms of hydrocarbons are aliphatic (straight- or branched-chain carbon arrangement) or aromatic (carbon arranged in a ring). Hydrocarbons are in many household and occupational products (Table 199-1). While all hydrocarbons can be toxic, aromatic and halogenated hydrocarbons are associated with the most severe systemic toxicity. Volatile agents are associated with the highest aspiration risk. Identification of the specific hydrocarbon or class can help anticipate specific potential toxicity and guide management.
| Hydrocarbon (state at room temperature) | Commercial Use |
|---|---|
| Aliphatic – linear structure, toxicity varies depending on volatility | |
| Gasoline (petrol) – liquid | Motor fuel |
| Kerosene (paraffin) – liquid | Stove and lamp fuel |
| Mineral seal oil – liquid | Furniture polish |
| Petroleum ether – liquid | Industrial solvent |
| Diesel fuel – liquid | Motor fuel |
| n-Hexane – liquid | Plastic cement, rubber cement |
| Methane, butane, propane, and ethane – gas | Fuel |
| Mineral spirits (white spirits) – liquid | Solvent, paint thinner |
| Turpentine – liquid | Solvent, paint thinner |
| Mineral oil (liquid paraffin) – liquid | Lubricant, laxative |
| Paraffin wax – solid | Industrial uses, candles |
| Petroleum jelly (petrolatum or soft paraffin) – solid | Skin lotion |
| Aromatic – ring structure, high toxicity | |
| Benzene – liquid | Chemical intermediate, gasoline (small amount, 0.8% on average) |
| Toluene – liquid | Airplane glue, plastic cement, acrylic paint |
| Xylene – liquid | Solvent, cleaning agent, degreaser |
| Halogenated – high toxicity | |
| Carbon tetrachloride – liquid | Solvent, refrigerant, aerosol propellant |
| Chloroform – liquid | Solvent, chemical intermediate |
| Methylene chloride – liquid | Paint stripper, varnish remover, aerosol paint, degreaser |
| Trichloroethylene – liquid | Spot remover, degreaser, typewriter correction fluid |
| Trichloroethane – liquid | Spot remover, degreaser, typewriter correction fluid |
| Tetrachloroethylene (perchloroethylene) – liquid | Dry cleaning agent, degreaser |
Chain length and branching determine the phase of the hydrocarbon at room temperature. Short-chain aliphatic compounds (up to 4 carbons), such as methane, ethane, propane, and butane, are gases; intermediate-chain aliphatic compounds (5 to 19 carbons), such as solvents, lamp oil, lighter fluid, and gasoline, are liquid; and long-chain aliphatic compounds (>19 carbons), such as waxes, are solids. Liquid hydrocarbons account for most exposures seen in the ED.1
Most hydrocarbon exposures occur as liquid ingestions or inhalations and usually have a benign clinical course.1,2 Serious toxicity and deaths associated with hydrocarbon exposure are usually due to ingestions rather than inhalation. Symptoms and signs of pulmonary injury develop in up to 50% of the children who ingest hydrocarbons,3,4 and hydrocarbon aspiration can produce acute respiratory distress syndrome.5 Suicidal injection of gasoline or kerosene with severe multiorgan toxicity has been reported.6,7
Volatile substances, usually hydrocarbon solvents contained in household or commercial products, can be inhaled for their euphoric effects (Table 199-2).8 Abusers are typically teenagers and younger adults, especially those in lower socioeconomic groups.9 Inhalation occurs by three different methods: (1) in “huffing,” the individual soaks a rag with the inhalant and then places it over the mouth and nose; (2) in “bagging,” the individual puts the hydrocarbon in a bag (usually a plastic bag) and repeatedly inhales deeply from the bag; and (3) in “sniffing,” the hydrocarbon is directly inhaled via the nostrils.10 In addition to causing deaths, abuse of volatile agents is associated with crimes such as homicide, sexual assault, and child abuse.11 The most commonly abused volatile hydrocarbons are paints, solvents, and gasoline.
| Product | Volatile Agent |
|---|---|
| Acrylic spray paint | Toluene |
| Adhesives, glue | Toluene, trichloroethylene |
| Aerosol propellants | Propellants and butane |
| Cigarette lighter refills | Butane |
| Degreasing agents | Trichloroethylene |
| Dry cleaning agents | Tetrachloroethylene |
| Fire extinguishers | Bromochlorodifluoromethane |
| Inhalational anesthetics | Nitrous oxide, halothane |
| Lighter fluid | Naphtha |
| Motor fuel | Gasoline (petrol) |
| Nitrites (“poppers”) | Isobutyl nitrite, amyl nitrite |
| Paint stripper | Methylene chloride |
| Plastic modeling cement | Methyl ethyl ketone, toluene |
| Spot removers | Trichloroethylene, trichloroethane |
| Typewriter correction fluid | Trichloroethane, trichloroethylene |
PATHOPHYSIOLOGY
The toxic potential of hydrocarbons depends on their physical characteristics (viscosity, surface tension, and volatility), chemical characteristics (aliphatic, aromatic, or halogenated), presence of toxic additives (pesticides or heavy metals), routes of exposure, concentration, and dose. The physical characteristics contribute the most to aspiration risk.
Viscosity refers to the general “thickness” of a liquid; fluids with a lower viscosity flow more easily than ones with high viscosity. Viscosity is measured in Saybolt universal seconds (SUS); fluids such as gasoline, kerosene, mineral seal oil, and turpentine have low viscosity (<60 SUS), whereas diesel fuel, grease, mineral oil, paraffin wax, and petroleum jelly have high viscosity (>100 SUS).12 Surface tension refers to the property where liquid molecules tend to cohere to each other. Liquids with high surface tension in contact with a solid surface tend to ball up, creating the smallest surface area rather than spreading out. Volatility refers to the ability of the liquid or solid to vaporize and is inversely related to the boiling point; highly volatile liquids have a low boiling point. Ingestion of liquids with low viscosity and surface tension and high volatility increases the risk for aspiration because these substance can flow easily, spreading out widely on the oral mucosa, and vaporize at body temperature. Inhalation of aromatic hydrocarbons or halogenated hydrocarbons can result in systemic absorption and the potential for significant toxicity.
CLINICAL FEATURES
Ingestion or aspiration of hydrocarbons mainly impairs the pulmonary system, but depending on the specific compound, the central nervous, peripheral nervous, GI, cardiovascular, renal, hepatic, dermal, and/or hematologic systems may be affected (Table 199-3).13
| System | Clinical Manifestations |
|---|---|
| Pulmonary | Tachypnea, grunting respirations, wheezing, retractions |
| Cardiac | Ventricular dysrhythmias (may occur after exposure to halogenated hydrocarbons and aromatic hydrocarbons) |
| Central nervous | Slurred speech, ataxia, lethargy, coma |
| Peripheral nervous | Numbness and paresthesias in the extremities |
| GI and hepatic | Nausea, vomiting, abdominal pain, loss of appetite (mostly with halogenated hydrocarbons) |
| Renal and metabolic | Muscle weakness or paralysis secondary to hypokalemia in patients who abuse toluene |
| Hematologic | Lethargy (anemia), shortness of breath (anemia), neurologic depression/syncope (carbon monoxide from methylene chloride), cyanosis (methemoglobinemia from amine-containing hydrocarbons) |
| Dermal | Local erythema, papules, vesicles, generalized scarlatiniform eruption, exfoliative dermatitis, “huffer’s rash,” cellulitis |
Hydrocarbon aspiration causes chemical pneumonitis by direct toxicity to the pulmonary parenchyma and alteration of surfactant function. Destruction of alveolar and capillary membranes results in increased vascular permeability and edema. The clinical manifestations of pulmonary aspiration are usually apparent soon after exposure from irritation of the oral mucosa and tracheobronchial tree. Symptoms include coughing, choking, gasping, dyspnea, and burning of the mouth. Patients with these symptoms should be assumed to have aspirated.
Signs include tachypnea, grunting respirations, wheezing, or retractions depending on the severity of aspiration. An odor of the hydrocarbon may be noted on the patient’s breath. Hyperthermia of ≥39°C (≥102.2°F) is likely and may occur initially or 6 to 8 hours after exposure. The fever is usually an inflammatory response due to pneumonitis. Necrotizing pneumonitis and hemorrhagic pulmonary edema may develop within minutes to hours in patients with severe aspiration. In most fatalities, these complications occur rapidly. With less severe damage, symptoms usually subside within 2 to 5 days, except in the case of pneumatoceles and lipoid pneumonias, the symptoms of which may persist for weeks to months.
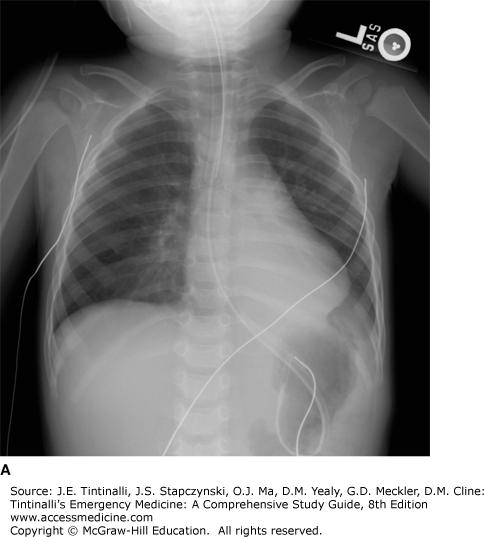
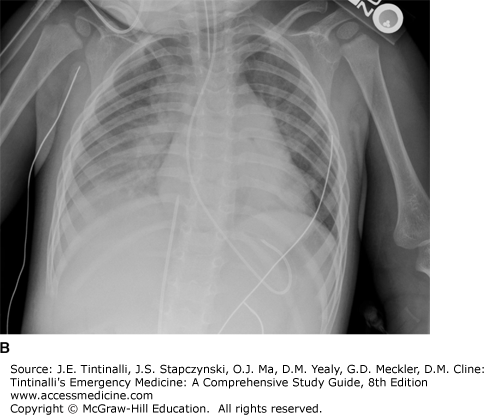
Although in most patients with clinically significant aspiration chest radiographic results eventually are abnormal, the time course of radiographic changes varies, and correlation with physical examination findings may be poor. Changes may be seen as early as 30 minutes after aspiration, but the initial radiograph in a symptomatic patient may be deceptively clear. Conversely, an asymptomatic patient can still have abnormal chest radiographic findings later during the clinical course. Radiographic changes usually appear by 2 to 6 hours and are almost always present by 24 hours, if they are to occur (Figure 199-1). The most common radiologic finding is bilateral infiltrates at the bases with multilobar involvement more common than single-lobe involvement and right-sided involvement more common than left-sided involvement.14,15,16 Hydrocarbon-induced aspiration pneumonitis can lead to lung necrosis and the creation of a pneumatocele.17
Life-threatening dysrhythmias, such as ventricular tachycardia and ventricular fibrillation, may occur with systemic absorption. Dysrhythmias occur most commonly after exposure to halogenated hydrocarbons and aromatic hydrocarbons. Exposure to short-chain aliphatic hydrocarbons occasionally causes dysrhythmias (ventricular fibrillation).10 The most worrisome acute complication found in solvent abusers is “sudden sniffing death syndrome” and occurs within minutes of exposure.8
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree