Chapter 60 HIV and the acquired immunodeficiency syndrome
HIV REPLICATION
Human immunodeficiency viruses (HIVs) 1 and 2 are retroviruses of the Lentivirus group. Like other lentiviruses, they exhibit tropism for cells of the immune system and cause immunological disorders, particularly immunodeficiency. Infection of immune cells in the nervous system may also cause neurological disease. Entry of HIV into cells of the immune system is via cell surface receptors. The major receptor is the CD4 molecule but the chemokine receptors CCR5 and CXCR4 play an important role as co-receptors. Inside the cell, the viral RNA is reverse-transcribed into DNA by a viral reverse transcriptase enzyme, and the DNA is integrated into the DNA of the host cell as proviral DNA by a viral integrase enzyme. The proviral DNA remains there until the cell is activated, when it is transcribed into RNA, which provides the template for assembly of new HIVs under the control of viral enzymes such as proteases. Budding of new virus from the cell is followed by infection of new cells and a repeat of the replication cycle.
PRIMARY HIV INFECTION
Initial infection by HIV-1 is associated with a primary HIV infection syndrome (also known as a seroconversion illness) in 50–70% of patients. This syndrome is similar to infectious mononucleosis, being characterised by fever, lymphadenopathy, headache, photophobia, fatigue and myalgia. However, mucocutaneous lesions, neurological disease and even transient immunodeficiency may also occur and, when present, this differentiates it from infectious mononucleosis. It may take a number of weeks for HIV antibody (detected by enzyme-linked immunosorbent assay (ELISA)) to become positive during seroconversion; however the level of plasma HIV RNA is very high. The diagnostic test of choice during this period is the measurement of HIV viral load utilising HIV RNA RT/PCR (reverse transcription of HIV RNA and amplification by polymerase chain reaction). HIV RNA peaks at a median of 10 days.1
DIAGNOSIS
A diagnosis of HIV infection can usually be made by demonstrating anti-HIV antibodies in the patient’s serum. However, the serological diagnosis of HIV infection can sometimes be problematic. A small minority of individuals who are not infected by HIV have serum antibodies which are reactive with some HIV proteins, and give false-positive results with some ELISAs. Many laboratories use two different types of ELISA to identify such sera. To ensure that HIV infection is not incorrectly diagnosed, an antibody test should only be considered positive if antibody is also detected according to defined criteria, using a confirmatory antibody test such as a Western blot immunoassay.2 Anti-HIV antibodies may be absent from the serum of patients with primary HIV infection. They are usually detectable by 2–6 weeks after infection, and almost always detectable by 12 weeks. After this time, absence of HIV antibodies excludes HIV infection in all but the most advanced cases of AIDS, or in a patient with an antibody deficiency disorder.
VIROLOGICAL MONITORING
HIV VIRAL LOAD MEASUREMENT
The major advance in viral diagnostics has been the development of polymerase chain reaction (PCR) and the application of this to the detection and quantification of viral nucleic acid in body fluids. Measurement of the blood ‘HIV load’ can be done by quantitating HIV RNA in plasma. Knowledge of the HIV load is useful in prognostication and essential when making decisions to commence and optimise therapy3 (Figure 60.1).
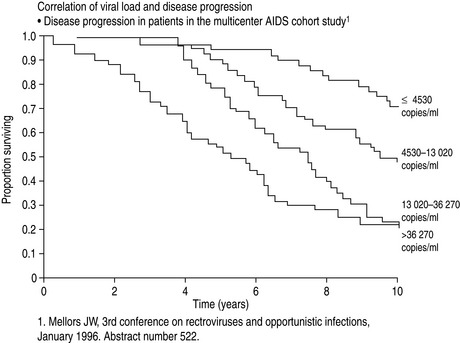
Figure 60.1 Correlation of viral load with disease progression. AIDS, acquired immunodeficiency syndrome.
(From Mellors JW, Rinaldo CR, Gupta P et al. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 1996; 272: 1167–70. Copyright: American Association for the Advancement of Science; reproduced with permission.)
GENOTYPING HIV TO DETECT DRUG RESISTANCE MUTATIONS
Drug susceptibility testing of HIV from a clinical specimen is performed in order to detect mutations in the genome of HIV that predict failure of individual antiviral therapeutic agents. The predominant genotype from an individual’s plasma or cerebrospinal fluid sample is determined by sequencing the reverse transcriptase and protease genes of complementary DNA (cDNA) obtained following RT/PCR. This information is used to assist the clinician and patient in the choice of an antiretroviral drug combination with the best chance of successful suppression of HIV replication, especially where resistance is suspected, i.e. in a failing regimen.4
IMMUNOLOGICAL MONITORING
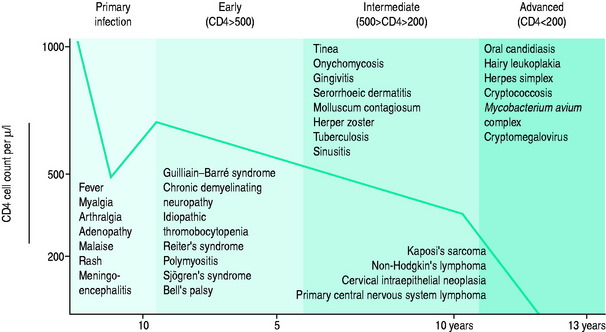
The blood CD4+ T-cell count or percentage is the best indicator of the severity of HIV-induced immunodeficiency and, therefore, of the patient’s susceptibility to opportunistic infections. Measurement of the blood CD4+ T-cell count or percentage is therefore critical in determining if the symptoms of an HIV-infected patient are likely to be caused by an opportunistic infection, and if so, what type of infection (Figure 60.2; and see text below).5
MANAGEMENT OF THE HIV-INFECTED PATIENT
The impact of combination antiretroviral therapy on the morbidity and mortality associated with HIV infection has been dramatic. The HIV Outpatient Study demonstrated a stepwise reduction in opportunistic infections and mortality with increasing intensity of antiretroviral therapy.6 In Australia, a comparison of cohorts before and after the introduction of combination antiretroviral therapy documented the effectiveness of these agents in reducing the risk of progression to AIDS and death.7 This refiects similar epidemiological studies conducted in Switzerland,8 France9 and the USA.10 A 70–80% reduction in mortality over 5 years has been the norm. AIDS-defining illnesses in Australia now occur predominantly in those without a past diagnosis of HIV infection.
Six classes of antiretroviral drugs are currently in use (Table 60.1). Fusion inhibitors block fusion of the virus with the cell membrane and CCR5 inhibitors block binding of CCR5-tropic strains of virus with CCR5, a co-receptor for HIV. Reverse transcriptase inhibitors are of three types. Firstly, nucleoside analogues and nucleotide analogues act by substituting for natural nucleosides or nucleotides during HIV replication, thereby inhibiting DNA chain elongation and the effects of the reverse transcriptase enzyme. Secondly, non-nucleoside reverse transcriptase inhibitors inhibit the reverse transcriptase enzyme by a different mechanism. Thirdly, integrase inhibitors block integration of viral DNA into host DNA and protease inhibitors inhibit the viral protease. All antiretroviral drugs have a limited duration of efficacy if used alone because HIV eventually develops resistance to them. The use of drug combinations is much more effective than single drugs, partly because drug resistance develops more slowly. Adherence to antiretroviral therapy is critical for the success of treatment.
Table 60.1 Antiretroviral drugs used to treat human immunodeficiency virus (HIV) infection
| Fusion inhibitors |
| Enfuvirtide (T20) |
| CCR5 inhibitors |
| Maraviroc |
| Nucleoside/nucleotide analogue reverse transcriptase inhibitors |
| Nucleoside analogues |
| Abacavir (ABV) |
| Didanosine (ddI) |
| Emtricitabine (FTC) |
| Lamivudine (3TC) |
| Stavudine (d4T) |
| Zidovudine (AZT) |
| Nucleotide analogues |
| Tenofovir (TNF) |
| Nucleoside/nucleotide fixed-dose combination tablets |
| Combivir (AZT + 3TC) |
| Trizivir (AZT + 3TC + ABV) |
| Kivexa (ABV + 3TC) |
| Truvada (TNF + FTC) |
| Non-nucleoside reverse transcriptase inhibitors |
| Delavirdine |
| Efavirenz |
| Nevirapine |
| Integrase inhibitors |
| Raltegravir |
| Protease inhibitors |
| Atazanavir* |
| Darunavir* |
| Fosamprenavir* |
| Indinavir* |
| Lopinivir* |
| Nelfinavir |
| Saquinavir* |
| Tipranavir* |
* Administered with low-dose ritonavir to increase serum levels (lopinavir + low-dose ritonavir are co-formulated as Kaletra).
DRUG TOXICITY
Recently, nucleoside analogue reverse transcriptase inhibitors (NRTIs) have been implicated in the development of syndromes that include fatigue, fat wasting, lactic acidosis and peripheral neuropathy. It has been suggested that these symptoms may be due to an inhibition of mitochondrial DNA (mtDNA) synthesis.11,12 Pancreatitis has occurred uncommonly with ddI (5–7%) and d4T (1–2%).
The most common adverse effect of NNRTIs is a skin rash. This occurs in up to 25% of subjects started on nevirapine and can range from a mild rash to Stevens–Johnson syndrome. Combination antiretroviral therapy is associated with a syndrome characterised by redistribution of fat (lipodystrophy) and fat atrophy (lipoatrophy). The biological mechanism responsible for the development of this syndrome is still unclear,13 although protease inhibitors in association with d4T have been implicated.
The use of antiretroviral therapy is also associated with hepatotoxicity in about 10% of patients.14 Co-infection with hepatitis C virus is one risk factor for hepatotoxicity and at least some cases are probably a type of immune restoration disease.15 Restoration of immune responses against pathogens also appears to be a cause of other types of inflammatory disease after the use of combination antiretroviral therapy.16,17
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree