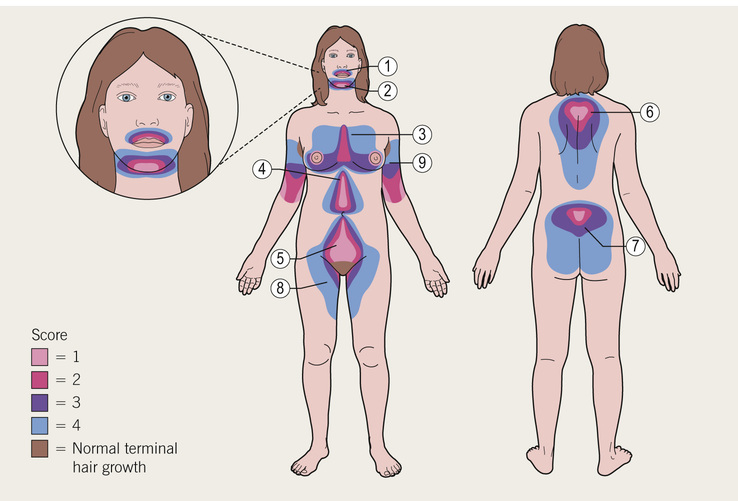
Michelle Freshman Hirsutism refers to excessive male pattern, terminal hair growth in women resulting from enhanced androgen-dependent sensitivity to increased levels of circulating androgens. Although areas of coarse, pigmented body hair are not unusual in women, concerns about abundance and distribution commonly arise. Thoughtful evaluation of the regions indicative of androgen excess helps discern a physiologic from pathologic cause. In each individual, regardless of gender or race, the number of hair follicles is predetermined (estimated to be 5,000,000); what differs are the pigmentation, thickness, phase duration, sebum production, and pattern of hair as driven by localized androgen responsivity1 at the pilosebaceous unit. In addition to peripheral androgen metabolism, insulin and hormonal fluctuations are thought to contribute.2 Hyperandrogenism may occur with or without ovulatory dysfunction or ovarian morphology abnormalities. When signs of virilism such as temporal balding or voice deepening accompany new-onset hirsutism, an ovarian, adrenal, or exogenous hormone source should be suspected, particularly in postmenopausal women as well as in those at increased risk of malignancy. Millions of women worldwide are affected by hirsutism. In reproductive-age women, 3% to 15% are considered hirsute. Well over 80% of hirsute women have hyperandrogenism. The most common diagnosis associated with this finding is polycystic ovary syndrome (PCOS). In a publication of the Androgen Excess and PCOS Society involving 6281 women with PCOS, hirsutism was identified in 74.7%.3 Mild cases are distinguished from clinically significant and progressive ones. In cases of isolated, mild hirsutism, approximately half appear to be unrelated to increased androgen levels, perhaps because of the limits of androgen detection or pilosebaceous level phenomena.4 The degree of clinical hirsutism does not correlate directly with elevated androgen level. This incongruity between symptoms and signs is seen in cases of idiopathic hyperandrogenism (6% to 15%),5 characterized by hirsutism in the setting of hyperandrogenism with normal ovulatory cycles and ovarian morphology as opposed to idiopathic hirsutism (4% to 7%),5 characterized by hirsutism in the setting of normal androgen levels, menses, and ovaries, thought to arise from normal ethnic or familial hair pattern variations. In addition, 8% to 13%3 of women report postmenopausal hirsutism, for which the pathophysiology is yet unclear.1 PCOS affects 4% to 20% of menstruating women.5,6 It is a heterogeneous syndrome, with varying contributions of excess androgens, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and insulin resistance.6 Most patients have a gonadotropin-dependent functional ovarian hyperandrogenemia, less often in conjunction with a mild adrenocorticotropic hormone (ACTH)–dependent functional adrenal hyperandrogenemia. Approximately 80% of patients are anovulatory2; 90% or more women with oligomenorrhea or amenorrhea have PCOS, and 95% of those with PCOS have oligomenorrhea or amenorrhea.7 The combination of PCOS and amenorrhea is more likely to produce severe hyperandrogenemia.7 In the mildest form of PCOS, women have neither gonadotropin nor ovulatory abnormalities but still have decreased sex hormone–binding globulin (SHBG) from ovarian stimulation. In more severe cases, insulin resistance and menstrual cycle irregularities may drive up androgen levels both through increased LH pulse frequency and because free testosterone cannot bind to as many sites as a result of reduced levels of circulating SHBG. One unifying, possibly initiating component, is thought to be ovarian theca cell abnormality leading to increased testosterone and/or androstenedione production and amplification of pituitary LH, wherein the progesterone negative feedback loop is impaired and LH is overproduced.6 In 2013 the Clinical Guideline Subcommittee of the Endocrine Society appointed a task force to formulate PCOS guidelines with oversight from subject experts of both the Endocrine Society and the European Society of Endocrinology.8 The resulting consensus requires two of the following: androgen excess (clinical or biochemical) and either ovulatory dysfunction (clinical) or polycystic ovaries (12 or more follicles 2 to 9 mm in diameter or >25 follicles per ovary and ovarian volume >10 mL in either ovary),6,8 excluding prepubertal and postmenopausal women. In practice, both clinical and biochemical assessments are subject to limitations. Whereas hirsutism correlates well with hyperandrogenemia, acne, alopecia, acanthosis nigricans, and skin tags do not.7 Cardiovascular risk indicators as well as metabolic disorders in hirsute women seem to correlate directly with androgen elevations.2 An important consideration for health care providers is deciding who is at risk for rare but potentially life-threatening conditions. Patients with PCOS are more likely to be obese, insulin resistant, and infertile9 as well as face cardiac sequelae. Although many hirsute women have PCOS (72% to 82%,),9 a minority (1.5% to 10%)9–11 have hyperandrogenic insulin-resistant acanthosis nigricans (HAIRAN) syndrome, late-onset nonclassic congenital adrenal hyperplasia (NCCAH), prolactinemia, a thyroid disorder, or an androgen-secreting neoplasm. Tumors of the ovary or adrenal gland comprise fewer than 0.2% of women with hyperandrogenemia, half of which are malignant.5,9 Hair follicles exist in all regions of the body except the lips, palms, and soles. Starting several months after birth, hair may be vellus—short and soft—or terminal—thicker, stiffer, longer, and darker. Hair development follows a prescribed, repeating pathway of “growth, regression, and remodelling events,”2 otherwise known as the anagen (active), telogen (static), and catagen (shedding) phases. Lengthening and thickening of vellus hair into terminal hair in androgen-sensitive but typically male-pattern regions of a woman’s body heralds hirsutism. Testosterone prolongs the anagen phase of terminal hair but shortens this phase in scalp hair.10 Vellus hair stems from undeveloped sebaceous glands.2 Distinguished from hirsutism is hypertrichosis. Despite an abundance of typically fine, short, unpigmented hair, androgens are not involved. This type of hair growth results from conditions such as anorexia nervosa, hypothyroidism, porphyria cutanea tarda, dermatomyositis and paraneoplastic syndrome, which causes hypertrichosis lanuginosa. Furthermore, cyclosporine, dexamethasone, diazoxide, minoxidil, penicillamine, phenytoin, and streptomycin are known contributors.12 Body hair is often diffusely distributed about the midline, including the face and even the forehead in a nonsexual pattern. In familial constitutional hypertrichosis totalis (Ambras syndrome), genetic factors, rather than disease, dictate growth pattern. A diagnosis of hypertrichosis can be assessed by hair type and distribution. Hirsutism and virilism result from greater testosterone activity. Testosterone activates the skin and hair follicles through enzyme type 1 5α-reductase, which converts testosterone to its potent metabolite, dihydrotestosterone (DHT). DHT converts vellus to terminal hair. This enzyme makes the difference, given similar androgen profiles, between a woman who is hirsute and one who is not. It is a process mediated by genetic variations.5 Adrenarche, marked by terminal hair growth in the axillae and on the lower pubic triangle, arms, and lower leg, results from increasing dehydroepiandrosterone sulfate (DHEA-S) concentrations. Most centrally located terminal body hair responds to sex hormone production, especially the amount, duration of exposure, and genetics, which together determine the resultant density and diameter. The number of hair follicles is said to decline after the age of 40 years. Acne may surface during puberty with the increased production of androgens and sebum. Adult women who develop hirsutism are clinically expressing hyperandrogenemia—that is, despite comparable total testosterone and DHT levels, they have different tissue-sensitive androgen metabolism. Terminal hair expression is ultimately controlled by follicular sensitivity to 5α-reductase. Serum testosterone and its immediate precursors—androstenedione, dehydroepiandrosterone (DHEA), and DHEA-S—are ultimately excreted through urine as 17-ketosteroids. These circulating androgens have a local influence at the pilosebaceous unit. DHEA-S mostly comes from the adrenal glands. The other two prohormones come from the peripheral conversion of circulating weaker androgens produced by the ovaries and adrenals. Women with PCOS have abnormal adrenocortical secretion, although only 25% have absolute adrenal excess.13 SHBG levels decrease because of suppressed liver production in response to one of several conditions: hyperinsulinemia, obesity, excess growth hormone (GH), or glucocorticoids. Hyperinsulinemia stimulates testosterone synthesis through ovarian thecal cells (hyperthecosis) acted on by LH. Hyperinsulinemia is also related to obesity (body mass index [BMI] >30 kg/m2) and obesity-related disorders (see Chapters 206 and 212). The correlation among high BMI (>27 kg/m2), PCOS, and androgen excess has been examined. Insulin resistance was more likely to occur in obese PCOS patients, but gonadotropins LH, androstenedione, DHEA, and DHEA-S were significantly higher in low-BMI PCOS patients than in weight-matched controls.13 Moreover, testosterone level was independent of BMI.13 Androgen abnormalities causing hirsutism and occasionally virilism include those found in NCCAH, acromegaly (a rare nonandrogenic cause related to GH excess), and Cushing syndrome with or without virilization caused by tumor (see Chapters 204 and 205). Serum DHEA and DHEA-S levels are highest in adrenal carcinoma and lowest in adrenal adenoma. Serum DHEA level varies directly with ACTH excretion, so a decrease in ACTH corresponds with a decrease in DHEA. Cases in which the ACTH is high but the DHEA and DHEA-S are low are attributed to Cushing syndrome or ectopic ACTH syndrome, seen without adrenal growths. SHBG is also diminished in hypothyroidism, although hypothyroidism is more likely to lead to hypertrichosis (see Chapter 214). In NCCAH an enzyme deficiency of 21-hydroxylase (21-OH), associated with the CYP21A2 gene, causes an accumulation of 17-hydroxyprogesterone (17-OHP). Two other, rarer congenital adrenal hyperplasia (CAH) deficiency syndromes associated with virilization are 11β-hydroxylase (CYP11B1 gene mutation) and 3β-hydroxysteroid dehydrogenase deficiency.12 Establishing a baseline is important because prior hair removal will render an objective assessment of hair growth pattern inaccurate. Furthermore, chronic skin irritation might lead to hair coarsening because of local changes at the pilosebaceous unit. Constitutional or familial hirsutism is common in individuals of Mediterranean or Middle Eastern descent but far less common in Asians. Inquiring about familial hair growth, acne, menstrual abnormalities, diabetes mellitus, hyperlipidemia, early-onset cardiac disease, maternal obesity, CAH, and a cancer diagnosis3 as well as prior diagnosis or treatment of hirsutism will serve in the initial screening. Gonadal abnormalities are indicative of elevated androgen levels and can be ascertained by a history of abnormal sexual development. Prepubertal androgenism or hermaphroditism might relate to CAH seen in early adrenarche or with adrenal tumors. Further workup is essential if signs of precocious puberty, clitoromegaly, fourchette development, or gonadal hypospadias are described. New-onset nipple discharge in a nonlactating woman is a key finding. A menstrual history and menopausal status should be elicited. Prepubertal or peripubertal stress causing an exaggerated adrenarche may be a contributing factor to cortisol overproduction and risk of PCOS.11 Infertility may point to anovulation or oligo-ovulation. Irregular or intermenstrual bleeding may indicate the presence of endometrial neoplasm. Exogenous hormone use may also play a role, whether for menstrual cycle control, acne management, or perceived competitive advantage among athletes. Thyroid replacement therapy can lead to manifestations of overtreatment, a rare cause of hirsutism. Hot or cold intolerance, sluggishness, tremors, hair thinning, weight gain, skin texture changes, and depression would suggest hypothyroidism. Hyperhidrosis; enlarged hands, feet, and face; and deepened voice might point to acromegaly. Patients with PCOS or Cushing syndrome are likely to have lipid and insulin abnormalities to differing degrees, even metabolic syndrome. Cushing syndrome is evidenced by excess ACTH from unsuppressed androgen. Mood or sleep changes, weight gain, glaucoma, osteoporosis, increased susceptibility to illness, weakness of upper arms, and signs of steroid excess in facies or skin might be seen with Cushing syndrome. By contrast, excessive thirst or appetite might point to glucose intolerance. Dyslipidemia and hypertension are common in PCOS; depression, sleep apnea, and endometrial carcinoma have also been associated with PCOS. Visual changes or headache could relate to a pituitary tumor. Finally, surgical history related to endocrine or metabolic disease management may be relevant. Weight, height, and vital signs are especially pertinent given the profile of a significant proportion of patients with PCOS. Significant weight gain is important in consideration of ACTH excess. Obesity is seen in patients with PCOS or Cushing syndrome (see Chapter 206), although Cushing syndrome may show up as a mixture of the following, depending on severity: central obesity, extremity or muscle wasting, red striae, supraclavicular fat pads, moon facies, thin skin or bruising, and sometimes a buffalo hump. A rise in blood pressure and concomitant hirsutism could raise suspicion for excess ACTH in Cushing syndrome or acromegaly, although acromegaly is not commonly associated with hirsutism. Acanthosis nigricans, a pigmented patch on the back of the neck, elbows, knuckles, knees, or intertriginous regions, is seen commonly in obese women and is indicative of insulin resistance. Thickened facial features, frontal bossing, prognathism, visual field defects, enlarged hands and feet, hyperhidrosis, or macroglossia may indicate the rare case of acromegaly. Thyroid enlargement or goiter should be noted and an abdominal examination performed to exclude masses. Typically, androgen excess is thought to include hirsutism, acne, and male pattern alopecia. PCOS might include skin tags.8 On the other hand, general hair loss might relate to hypothyroidism. An essential component of the physical examination must include an objective measurement of hair growth pattern and quantity. Such an assessment has been captured by the standard Ferriman-Gallwey scoring system, originally established in 1961,1 as well its modified form by others (the mFG; Fig. 207-1). The mFG serves to establish hirsutism clinically by rating nine body areas on a scale of 0 to 4 (upper lip, chin, chest, upper and lower back, upper and lower abdomen, arm, and thigh, excluding the original forearm and lower leg, which contribute less because these areas are overly androgen sensitive) (see Fig 207-1). Limitations of the mFG include poor inter-rater reliability and lack of sensitivity in the composite to heavily weighted areas that are particularly affected, such as on the face, lack of adjustments for racial variations, and the failure to assess the patient’s perception of unwanted hair. A score higher than 8 in nonblack, non-Southeast or non–Far East Asians3 indicates moderate hirsutism; a score higher than 15 indicates moderate to severe hirsutism and is an independent risk for PCOS.9 Terminal hair on the upper back, shoulders, upper chest, cheeks, or upper abdomen delineates male pattern hair growth; masculinization involves huskiness of the voice, increased pectoral muscle mass, balding, mammary atrophy (defeminization), and, in cases of virilization, a fully deepened voice, changes in libido, and clitoromegaly.14 Sexual ambiguity—such as downward placement of the urethral meatus or joining of posterior labial folds—may denote a congenital defect. A pelvic examination is performed to assess for the presence of ovarian masses. Pregnant women may need careful monitoring in the face of gestational hirsutism; postpartum patients and their newborns are observed for regression of virilization, once malignant causes have been excluded.1
Hirsutism
Definition and Epidemiology
Polycystic Ovary Syndrome
![]() Specialist referral is indicated for all patients with suspected hirsutism.
Specialist referral is indicated for all patients with suspected hirsutism.
Pathophysiology
Clinical Presentation
Physical Examination
Hirsutism
Chapter 207