Alteration in cardiac preload and afterload conditions
Negative inotropic effect
Depressed autonomic nervous system activity
Vasodilation
Depressed sympathetic nervous system activity
Decreased functional residual capacity
Relief of pain, stress, anxiety
Decreased muscular activity
Decreased myocardial O2 demand (negative chronotropic and inotropic effect)
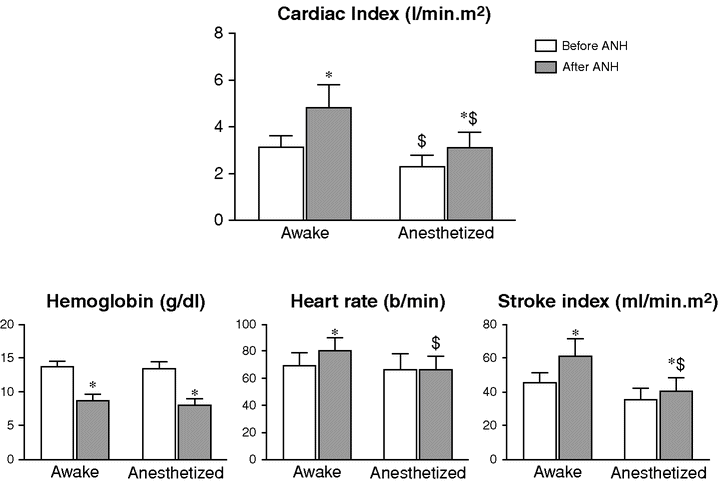
Effects of anesthesia on the physiological response to acute normovolemic hemodilution (ANH).
* p < 0.05 after ANH versus before ANH.
$ p < 0.05 anesthetized versus awake.
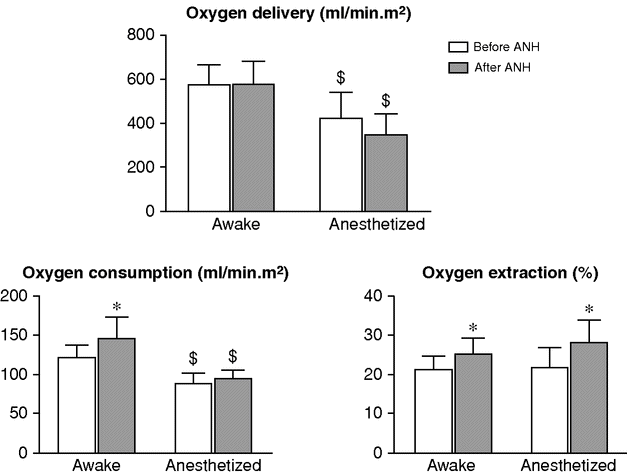
Effects of anesthesia on oxygen delivery, oxygen consumption, and oxygen extraction during acute normovolemic hemodilution (ANH).
* p < 0.05 after ANH versus before ANH.
$ p < 0.05 anesthetized versus awake.
Interestingly, ANH appears to be associated with increased oxygen consumption in awake patients, which could be related at least in part to an increase in myocardial oxygen demand. In the Ickx et al. study,[15] when the patients undergoing ANH while awake were anesthetized, all the measured parameters returned to values similar to those obtained in patients undergoing hemodilution while anesthetized. Therefore, performing ANH before or after induction of anesthesia did not result in a significant different physiological response at the time of surgery.
Limits of hemodilution
As described above, maintenance of tissue oxygenation during ANH results from an increase in cardiac output and oxygen extraction. Several experimental and clinical studies have demonstrated the involvement of both mechanisms even in the early stage of ANH.[16] The relative contribution of these mechanisms will depend on the ability of the organism to recruit them. They allow the maintenance of adequate tissue oxygenation until the hemoglobin concentration falls to about 3 to 4 g/dl (hematocrit 10–12%). Below this “critical” value, oxygen delivery can no longer match tissue oxygen demand, and cellular hypoxia will develop, as demonstrated in several experimental studies.[17–20] Van Woerkens et al., who studied a Jehovah’s Witness patient who died from extreme hemodilution, reported a critical hemoglobin concentration of 4 g/dl.[21]
The efficacy of the mechanisms maintaining tissue oxygen delivery when the oxygen-carrying capacity of the blood is reduced depends primarily on the maintenance of an adequate circulating blood volume. Indeed, hypovolemia blunts the effects of decreased blood viscosity on venous return.[22] Although “normovolemic” conditions are difficult to define, replacement of the blood and fluid losses with at least a volume of substitute having the same expanding effect on the intravascular volume is required. Only a few studies have compared the effects of different plasma substitutes on the hemodynamic response to ANH: colloids appeared superior to crystalloids.[23,24] On the one hand, compared with colloids, crystalloids have a very low volume-expanding effect in the context of ANH.[25] On the other hand, colloids and in particular tetrastarches appeared to have potential beneficial effects on renal tissue [26] and modulation of inflammation.[27]
Tolerance to acute isovolemic hemodilution not only depends on the integrity of the compensatory mechanisms described above, but also on the level of tissue oxygen demand. For a given cardiac output and oxygen extraction response, any increase in tissue oxygen demand will require a higher hemoglobin concentration.
Acute normovolemic hemodilution and the cardiac patient
Maintenance of myocardial oxygen delivery during ANH depends essentially on the increase in the coronary blood flow as oxygen extraction is already nearly maximal at the level of the heart under resting conditions.[8] This is achieved by a reduction in coronary vascular resistance related to the decreased blood viscosity and to specific coronary vasodilation. Heart rate and possibly myocardial contractility have been shown to increase during hemodilution,[28] which results in an augmentation of myocardial oxygen demand. When the hematocrit is reduced to about 10%, myocardial oxygen consumption more than doubles and coronary vasodilation is nearly maximal. Below such a hematocrit, coronary blood flow can no longer match myocardial oxygen demand and ischemia develops, ultimately resulting in cardiac failure.
The dependency of myocardial oxygen supply to the coronary blood flow highlights the vulnerability of the heart during ANH, especially in patients with coronary artery disease (CAD) in whom coronary blood flow cannot increase. The lowest tolerable hemoglobin concentration in CAD patients remains unknown.[29] A recent pilot study addressing specifically the problem remained inconclusive.[30] It probably depends on several factors, including the severity of the disease.[31] There is, however, increased evidence that tolerance of CAD patients to isovolemic anemia closely depends on the level of myocardial oxygen demand.
In anesthetized patients scheduled for coronary surgery, several studies demonstrated that moderate ANH (target hematocrit value 27–33%) is well tolerated, and may be even cardioprotective if associated with a decreased myocardial oxygen demand.[32] Myocardial oxygen balance is profoundly influenced by the level of heart rate, and recent clinical data confirm that tolerance of CAD patients to moderate anemia is closely related to the level of heart rate.[33] The anesthetic technique also may play a role.[34] The early postoperative period is certainly critical in hemodiluted CAD patients, because they have to face an increased tissue metabolic demand.[35]
Acute normovolemic hemodilution and hemostasis
Hemodilution could affect hemostasis in different ways. Firstly, it will dilute not only plasmatic factors, but also cellular coagulation factors, such as platelets and, of course, RBCs. Red blood cells have been shown to interfere with hemostasis through a mechanical effect but also through biological effects related to the release of intracellular adenosine diphosphate and to the generation of thrombin.[36] The clinical consequences (i.e. importance of perioperative bleeding) of these interactions between RBCs and hemostasis remain to be determined.
Hemodilution could also affect hemostasis through the direct effects of plasma substitution fluids on the platelets and the coagulation mechanisms.[37] These effects are more marked with colloids than with crystalloids. Among colloids, they are more marked with dextrans than with gelatins and albumin. For hydroxyethyl starches, these effects seem to be closely related to the intrinsic properties of the different solutions, such as a high in vitro molecular weight and a high degree of hydroxyethyl substitution.[38] Several in vitro studies have confirmed these effects in the context of ANH.[39,40] In addition, patients with Type O blood may demonstrate more coagulation compromise than those with non-O blood when undergoing hemodilution with low molecular weight hydroxyethyl starch.[41]
Storage of whole blood during ANH might be associated with disturbances in platelet aggregation,[42] although the clinical relevance of this observation remains unknown. Agitation during storage of ANH blood does not seem to have any effect on platelet function.[43]
Despite the fact that ANH may directly interfere with normal hemostasis, there is no evidence from the literature that ANH is associated with increased perioperative bleeding,[44,45] although it may affect standard coagulation tests.[46]
Efficacy
Theoretical aspects
The basic concept behind ANH is that patients undergoing such procedure will lose fewer erythrocytes per milliliter of lost blood during surgery and after transfusion of the collected autologous blood in the immediate postoperative period.[5]
Several equations have been developed to calculate the efficacy of ANH as a function of surgical blood loss, initial hematocrit, target post-ANH hematocrit, and hematocrit used as the transfusion trigger. Presuming a “usual” surgical patient without preoperative anemia, and a transfusion decision based exclusively on a trigger hemoglobin concentration of 6–7 g/dl, Weiskopf [47] calculated that 55 to 77% of patient’s total blood volume must be lost during surgery in order to achieve savings of about 180 ml of RBCs, which represents one standard blood unit. However, the usefulness of the different published equations in clinical practice remains limited, as several factors have not been always taken into account in the proposed formulae.[2].
Results from the literature
Although efficacy of ANH as a blood conservation technique remains highly debated,[44,45,48,49] this technique is still currently used in many adult and pediatric centers.[50,51] Most of the studies reviewed were performed in the setting of cardiac or orthopedic surgery. Adequate evaluation of the published results was hampered by the relative poor quality of the studies and the marked heterogeneity observed between trials, partly explained by study factors (patient populations, target hematocrit values, transfusion triggers, ANH technique, etc.). Efficacy of ANH was found to be relatively modest in terms of likelihood of exposure to allogeneic blood and units transfused. It closely depends on the use or not of protocols to guide transfusion practice. There was no obvious increase in adverse events with ANH, but the incidence of complications was poorly reported.
Studies that demonstrated a clear efficacy of ANH in reducing the likelihood of patient’s exposure to allogeneic blood in the perioperative period have been performed in major liver resection [52,53] or major abdominal surgery.[54] In all of them, a high volume of blood was collected. Surgery was associated with significant blood loss, and a low hemoglobin concentration (7–8 g/dl) was used as the transfusion trigger. Adequate selection of patients may also play a role.[55] All these observations indicate that efficient ANH requires significant expertise in the field from the care-giving team.
Conclusions
Acute normovolemic hemodilution entails the removal of blood from a patient shortly after the beginning of the surgical procedure, and its replacement with crystalloids or colloids to maintain the circulating blood volume. It is a relatively simple, cheap, and effective tool to avoid or reduce allogeneic blood transfusion. Factors that influence the efficacy of the technique have been clearly identified. This reduces the field of application of ANH to patients undergoing surgery with high bleeding risk in whom a great volume of blood can be collected.
Knowledge of the physiological compensatory mechanisms that occur during normovolemic hemodilution and their limits is essential for the safe use of the technique. In addition, the anesthesiologist must be familiar with its practical aspects. Although ANH has a place in different types of surgery, it must be regarded as an integral part of a blood conservation strategy tailored to the individual patient’s needs and adapted to specific surgical procedures.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




