Chapter 12 Haemodynamic monitoring
Haemodynamics is the study of blood flow. Haemodynamic monitoring therefore refers to the monitoring of blood flow through the cardiovascular system. In the intensive care unit (ICU), haemodynamic monitoring is used to detect cardiovascular insufficiency, differentiate contributing factors and guide therapy.
There has been debate over the risks and benefits of invasive haemodynamic monitoring in critically ill patients. Because there are scant data to show that invasive monitoring leads to improved survival, there has been a steady trend toward less invasive monitoring in the ICU. Regular clinical assessment remains an important component of haemodynamic monitoring, and any acquired physiological data must be interpreted in the clinical context. Importantly, monitoring must be combined with effective therapy in order to demonstrate an impact on outcome.1
ARTERIAL BLOOD PRESSURE
In the absence of sophisticated waveform analysis, arterial pressure is poorly correlated with cardiac output. Hence clinical estimation of cardiac output from arterial pressure and heart rate is unreliable until extreme hypotension occurs.2
NON-INVASIVE ARTERIAL BLOOD PRESSURE (NIBP) MEASUREMENT
Oscillometry overestimates low pressures and underestimates high pressures, but for the normotensive range the 95% confidence limits are ± 15 mmHg (2 kPa). Dysrhythmias increase the error. Cuff width should be 40% of the mid-circumference of the limb. Narrower cuffs overestimate and wider cuffs underestimate blood pressures.
INVASIVE BLOOD PRESSURE MEASUREMENT3
The radial artery is the most common site for cannulation. Nothing larger than a 20-gauge cannula is advisable, and either a modified Seldinger technique or direct cannulation can be used. Once inserted, the cannula is usually infused with normal or heparinised saline at 3 ml/h, with a snap flush rate of 30–60 ml/h. Although it appears not to prolong patency, the use of heparinised saline (2–4 units/ml) might improve accuracy.4 It should be avoided if heparin-induced thrombocytopenia syndrome is suspected.
The axillary, brachial, femoral, posterior tibial and dorsalis pedis arteries can all be used without apparently raising the complication profile (Table 12.1). In severe circulatory compromise, gaining peripheral arterial access may be difficult and time-consuming. Rapid femoral cannulation by the Seldinger percutaneous technique usually remains feasible, with the added advantage that femoral arterial monitoring more accurately reflects aortic pressure in low-output states.
Table 12.1 Complications of arterial cannulation, with suggested preventive and treatment options61
| Complication | Prevention | Treatment |
|---|---|---|
| Vascular thrombosis (ranges from 7 to 30% following radial artery cannulation). Risk factors for digital ischaemia include: shock, sepsis, embolus of air or clot, hyperlipoproteinaemia, vasculitis, female sex, prothrombotic states, accidental intra-arterial injection of drugs | Risks reduced by smaller catheter, larger artery, decreased duration of cannulation, avoiding traumatic insertion and multiple attempts. Allen’s test (including modifications such as Doppler, plethysmography and digital blood pressure) is probably unhelpful | Remove cannula. Arterial thrombosis is usually self-limiting. Severe ischaemic damage estimated at < 0.01%. Anticoagulation and/or vascular surgery/intervention may be necessary |
| Distal embolisation | Diligent catheter care and observation | As for thrombosis |
| Proximal embolisation of clot or air (can result in stroke) | Diligent catheter care and observation. Exclude air from pressurised system. Avoid axillary, subclavian or carotid access | Tailored to sequelae |
| Vascular spasm | Smaller catheter, larger artery. Avoid traumatic insertion and multiple attempts | Remove cannula. Resite if necessary |
| Skin necrosis at catheter site | Diligent catheter care and observation | Surgical debridement and skin grafting may be necessary |
| Line disconnection and bleeding/exsanguination | Minimise connections. Diligent catheter care and observation | Control bleeding. Transfusion may be necessary |
| Accidental drug injection | Clearly label arterial line near ports | Leave cannula in situ to facilitate treatment if required. Depends on drug injected. May require papaverine or procaine, analgesia, sympathetic block of limb and anticoagulation |
| Infection – local or systemic | Diligent catheter care and observation | Remove cannula and send tip for culture. Resite if necessary. Immobilise and elevate affected upper limb. Start empiric antibiotics if sepsis or septic shock is present |
| Damage to nearby structures such as nerves, directly or due to haematoma (e.g. compartment syndrome or carpal tunnel syndrome). Arteriovenous fistula. Femoral approach can be associated with bowel damage | Careful insertion technique. Seek assistance from experienced operator | Tailored to sequelae. Haematomas can develop into pseudoaneurysms requiring surgery |
Cannulae are usually removed or resited between days 5 and 7, or earlier if a complication is suspected. Distal perfusion should be checked at least 8-hourly, and the cannula removed if there is persistent blanching, coolness with sluggish capillary refill, loss of pulses or evidence of raised muscle compartment pressures. Complications associated with arterial cannulation are presented in Table 12.1.
PHYSICAL PROPERTIES OF CLINICAL PRESSURE MEASUREMENT SYSTEMS5,6
The natural resonant frequency of the system should ideally exceed 30 Hz (> 10 harmonics) for heart rates up to 180 beats/min (3 Hz) to prevent distortion of the biological signal by sine-wave system oscillations. Damping refers to any property of an oscillatory system that reduces the amplitude of oscillations. Factors that increase oscillation in the system, such as increased tubing length, diameter or compliance, cause underdamping. Overdamping tends to smooth the waveform, causing underestimation of SBP and overestimation of DBP, while MAP tends to be preserved. Contributing factors include clots, air bubbles and loose connections. Damping can be assessed clinically by the fast-flush test (Table 12.2).7
Table 12.2 Fast-flush test to assess dynamic response (damping) of the blood pressure-monitoring system
| Make a paper record of transducer output |
| Snap the valve of the continuous flush system. This produces a square wave on the output trace |
| Repeat the process at least twice |
| Resonant frequency is the distance between successive peaks divided by the paper speed in millimetres per second |
| Damping is satisfactory (critical damping) if each snap test has two to three oscillation waves, with each wave one-third or less the size of the preceding wave |
ADDITIONAL INFORMATION FROM THE ARTERIAL WAVEFORM
ESTIMATION OF STROKE VOLUME AND CARDIAC OUTPUT8
Estimation of stroke volume by analysis of the arterial pressure waveform, in particular various properties of the pulse pressure (pressure above diastolic) component, has been studied for many years. Pulse contour analysis calculates stroke volume from the area under the systolic portion of the arterial pressure waveform. After individual calibration, these monitors can determine stroke volume beat-to-beat. Cardiac output, derived by multiplying stroke volume by heart rate, appears to offer reliable continuous cardiac output monitoring, even during haemodynamic instability.9 However, accuracy in the presence of arrhythmia has not been validated.
Transpulmonary indicator dilution (discussed below) is the usual method of calibration. Pulse contour devices that use thermodilution or lithium ion calibration are commercially available.10 Comparisons with pulmonary artery catheter (PAC) cardiac output measurements have shown good agreement, with mean bias values ≤ 0.1 l/min and precision (SD of bias) of the order of 0.6 l/min.
Disadvantages of such techniques can include:
Recently developed proprietary algorithms continuously measure stroke volume and cardiac output without requiring calibration against another method.11 Additional clinical validation is awaited.
CENTRAL VENOUS CATHETERISATION (CVC)
CVC is indicated for monitoring of central venous pressure (CVP) and administration of certain drugs and parenteral nutrition. Modified catheters are also available which continuously monitor central venous oxygen saturation (ScvO2). Contraindications to CVC are relative and reflect potential complications of the procedure (Table 12.3). These should be considered in selecting the site for catheter insertion. Particular attention should be paid to the coagulation profile and factors which affect coagulation such as thrombolysis and activated protein C therapy.
Table 12.3 Complications of central venous catheterisation, with preventive and treatment options62
| Complication | Prevention | Treatment |
|---|---|---|
| Intravascular loss of guidewire | Ensure guidewire is always secured. Avoid retracting guidewire into insertion needle. Seek assistance from experienced operator | Interventional radiology or surgery may be required for retrieval |
| Air embolism | Proper patient positioning, including Trendelenburg (head-down tilt) for jugular or subclavian insertion. Consider alternative insertion sites. Ensure connections are tight | Left lateral Trendelenburg position. Administer 100% oxygen and ventilatory support. If catheter in place, tighten all connections and attempt to aspirate air. Basic/advanced life support if necessary |
| Dysrhythmias and conduction defects such as right bundle branch block | Continuous electrocardiographic monitoring during insertion. Correct placement of catheter tip | Withdraw or remove guidewire or catheter |
| Damage to nearby structures: Pneumothorax/haemothorax/chylothorax/hydrothorax, particularly with approaches to subclavian vein. Nerve injury such as phrenic, recurrent laryngeal, Horner’s syndrome. Arterial puncture – including injury to carotid, subclavian, aorta or pulmonary artery. Haematoma, pseudoaneurysm, arteriovenous fistula can result. Stroke may result from carotid artery injury. Tracheal injury. Femoral approach can be associated with bowel damage | Identify risk factors such as previous surgery, skeletal deformity or scarring at site of insertion. Seek assistance from experienced operator. Select insertion site depending on impact of potential complications. Consider ultrasound guidance. Scheduled, routine replacement of catheter increases risk of mechanical complications | Tailored to sequelae |
| Line disconnection and bleeding | Minimise connections. Proper catheter care and observation | Control bleeding. Transfusion may be necessary |
| Infection – local or systemic (including endocarditis) | Aseptic insertion technique. Lower risk with subclavian compared to internal jugular or femoral insertion. Antimicrobial-impregnated catheters. Disinfect catheter hubs. Routine scheduled resiting appears not to reduce systemic infection rates. Remove catheter when no longer required | Remove catheter; resite if necessary. Culture blood and catheter tip. Start empiric antibiotics if sepsis or septic shock is present |
| Superior vena caval erosion can result in haemothorax or cardiac tamponade | Remove catheter when no longer required | Early detection and surgical intervention |
| Thrombosis | Remove catheter when no longer required. Subclavian insertion lower risk than internal jugular or femoral | Anticoagulation, vascular or endovascular intervention may be required |
Traditionally, puncture of the central vein is performed by passing a needle along the anticipated line of the vein with reference to surface anatomical landmarks (landmark method). Catheterisation is usually achieved via the subclavian, internal jugular or external jugular veins into the superior vena cava.12 The median cubital and basilic veins are used less commonly. Access to the subclavian vein is usually via the infraclavicular approach, but the supraclavicular approach is safe and reliable in experienced hands.
The use of ultrasound has been advocated to optimise the success rate of CVC insertion and minimise complications.13 Two-dimensional imaging can be used to localise the vein and define anatomy prior to placement of a CVC by standard landmark techniques. Ultrasound can also provide real-time, two-dimensional guidance during CVC insertion. Alternatively, audio-guided Doppler ultrasound can aid localisation of the vein and differentiate it from its companion artery during CVC insertion.
In 2002, the UK National Institute for Clinical Excellence issued guidelines recommending the use of ultrasound for elective catheterisation of the internal jugular vein, and consideration of ultrasound guidance in most clinical situations.14 These recommendations for ultrasound-guided internal jugular catheterisation are supported by meta-analysis.15 However, there are limited data supporting its use for subclavian and femoral veins.
The right tracheobronchial angle and carina are common radiological markers of insertion depth. In an attempt to reduce further the incidence of venous erosion (which is rare but potentially catastrophic), some practitioners place the catheter tip lower in superior vena cava or even in the upper right atrium, ensuring that the catheter is parallel to the long axis of the vein so that the tip does not abut the vein or heart wall end-on.16
Femoral venous catheterisation with radiological positioning of the tip close to the right atrium produces pressure measurements in good agreement with the subclavian CVP.17
CENTRAL VENOUS PRESSURE
The normal CVP in the spontaneously breathing supine patient is 0–5 mmHg, while 10 mmHg is generally accepted as the upper limit during mechanical ventilation. In health there is a good correlation between CVP and pulmonary artery occlusion pressures (PAOP), but this is lost in many types of critical illness such as pulmonary hypertension, pulmonary embolism, right ventricular infarction, left ventricular hypertrophy and myocardial ischaemia. The relationship between CVP and right ventricular end-diastolic volume (RVEDV: preload) is altered in critical illness by changes in right ventricular diastolic compliance and juxtacardiac pressures.18
Except at extreme values, static measures of CVP do not differentiate patients likely to respond to fluid therapy from non-responders. However, dynamic changes in CVP either in response to volume loading or related to respiration can assist in evaluating volume status.19 For instance, a steep increase in CVP following volume challenge suggests the heart is functioning on the plateau portion of the Frank–Starling curve. Severe hypotension with a low or normal CVP is unlikely to be due to acute pulmonary embolism, cardiac tamponade or tension pneumothorax.
Although fluid manometry is sufficient to measure the CVP, the frequency response is low and waveform analysis impossible (Table 12.4). Therefore, an electrical transducer system is normally used.
Table 12.4 Analysis of central venous pressure waveform
| Condition | Pressure changes | Waveform changes |
|---|---|---|
| Tricuspid regurgitation | Increased RA pressure | Prominent v-wave, x descent obliterated, y descent steep |
| Right ventricular infarction | RA and RV pressure elevated. RAP does not fall and may rise in inspiration | Prominent x and y descents |
| Constrictive pericarditis | RA, RV diastolic, PA diastolic and occlusion pressures elevated and equalised. RAP may rise in inspiration | Prominent x and y descents |
| Pericardial tamponade | RA, RV diastolic, PA diastolic and occlusion pressures elevated and equalised. RAP usually falls in inspiration | y descent damped or absent |
RA, right atrial; RV, right ventricular; RAP, right atrial pressure; PA, pulmonary artery.
In a trend toward less invasive monitoring, peripheral venous pressure and non-invasive estimates of CVP have demonstrated potential as alternatives to direct CVP measurement.20,21
ScvO2
A recent prospective randomised study of patients with severe sepsis and septic shock demonstrated a survival advantage with the use of this monitoring technique and an emergency department treatment protocol (early goal-directed therapy).22 ScvO2 has been proposed as a marker of tissue hypoxia (see Chapter 14). It is mentioned here as an example of the scant data relating haemodynamic monitoring to improved survival.
PULMONARY ARTERY CATHETER
Right-heart catheterisation using a flow-directed balloon-tipped catheter was introduced by Swan and Ganz in 1970.23 The ability to monitor sophisticated haemodynamic and gas exchange variables at the bedside appealed to clinicians, and the PAC was rapidly accepted into routine critical care.
In 1996 a non-randomised cohort study of PAC use in American teaching hospitals appeared to show that, in any of nine major disease categories, PAC in the first 24 hours increased 30-day mortality (odds ratio 1.24, 95% confidence interval (CI) 1.03–1.49), mean length of stay and mean cost per hospital stay.24 A simultaneous editorial called for a moratorium on PAC use, and for a prospective multicentre trial.25
A subsequent Cochrane database systematic review of PAC monitoring in adult ICU patients incorporated data from two recent multicentre trials and 10 other studies.26 The pooled mortality odds ratio for studies of general ICU patients was 1.05 (95% CI 0.87–1.26) and for studies of high-risk surgery patients was 0.99 (95% CI 0.73–1.24). PAC monitoring had no impact on ICU or hospital length of stay (where reported). A recent multicentre trial incorporating protocolised haemodynamic management of patients with acute lung injury compared PAC-guided with CVC-guided therapy.27 There were no significant differences in 60-day mortality or organ function between groups. Overall, these data suggest that PAC monitoring in critically ill patients is not associated with increased mortality or with survival benefit.
On the other hand, recent data suggest a differential association between PAC use and mortality dependent upon severity of illness. Logistic regression analyses of data from a tertiary-care university teaching hospital28 and from the National Trauma Data Bank (American College of Surgeons)29 suggest that PAC use may be associated with survival benefit in the most severely ill/injured patients.
Overall, the PAC still finds application as a haemodynamic monitoring tool in the ICU and the operating room. However, its role is under increasing scrutiny, and continues to decline in favour of less invasive alternatives. It could be argued that future PAC use should be guided by studies to determine optimal management protocols and appropriate patient groups.26
Traditional indications for PAC monitoring have been to:
CATHETER INSERTION30
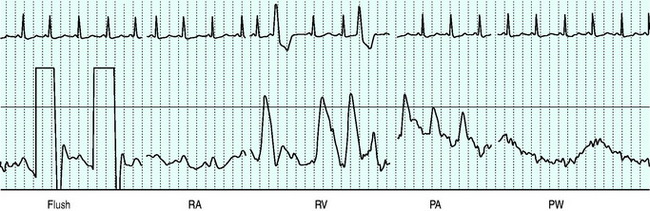
Balloon volume is 1.5 ml. The balloon should be tested prior to insertion into the sheath. Deflation should always occur passively to minimise the risk of balloon damage. Before passage through the heart the balloon should be inflated to assist flow guidance and protect against myocardial injury and dysrhythmias. Inflation should not be forced, and should not alter the waveform prior to wedging. The right atrium is reached at 15–20 cm from the internal jugular vein, 10–15 cm from the subclavian vein, 30–40 cm from the femoral vein and 40 and 50 cm respectively from the right and left basilic veins. The right ventricle and pulmonary artery (PA) are then entered at additional 10-cm intervals, with a further 10 cm to PA occlusion.31 Waveforms seen as the catheter floats to the wedged position are shown in Figure 12.1.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree