CHAPTER 64
Gynecologic Cancers
Renee McLeod-Sordjan, DNP, FNP-BC, Acute Care-BC
During the past decade, the role of human papilloma virus (HPV) in the pathogenesis of gynecologic cancer in both genders has been elucidated. HPV infection potentially causes approximately 11,000 cervical cancers, 4,800 non-cervical cancers in men, and 4,100 noncervical cancers in women (Chaturvedi, 2010). In 2014, an estimated 94,990 American women will be diagnosed with a gynecologic cancer with 28,790 succumbing to their illness (American Cancer Society [ACS], 2014). There are five main types of cancer that affect a woman’s reproductive system: cervical, ovarian, uterine, vaginal, and vulvar. A sixth rare type of gynecologic cancer, fallopian type, completes the spectrum of female gynecologic malignancies. Gynecologic malignancies represent 11% of all cancers affecting women (NCI, 2013; Schiffman et al., 2011). They are the fourth most common cancers in American women. Uterine cancers account for 55% of these tumors (ACS, 2014; NCI 2013). Yet cervical, endometrial, and ovarian malignancies contribute significantly to the morbidity and mortality of the female population. Whereas cervical and endometrial cancers can be detected early in their development, many patients with ovarian cancer present with already advanced disease. Early detection is essential for the greatest possibility of treatment and improved mortality.
This chapter reviews the five major female gynecologic cancers (cervical, vaginal, vulvar, endometrial, and ovarian) as well as trophoblastic disease, and addresses how primary care clinicians can increase primary prevention, overcome diagnostic challenges, and improve utilization of gynecologic cancer screening. In addition, the widespread use of prophylactic HPV vaccination in primary care may offer primary prevention for 70% of cervical cancer and 95% of noncervical cancer associated with HPV 16 and 18.
 CARCINOMA OF THE CERVIX
CARCINOMA OF THE CERVIX
Anatomy, Physiology, and Pathology
The cervix is a fusiform cavity that communicates below with the vagina and above with the uterine body. The epithelium of the upper two thirds is columnar, whereas the lower third gradually changes to squamous close to the external opening of the cervix (os). The transformation zone is a ring of tissue located at the squamocolumnar junction; where the squamous epithelium of the vagina meets and replaces the glandular epithelium of the endocervical canal and is the site of continuous metaplastic change. This change is most active in utero, at puberty, and during the first pregnancy, and then declines after menopause. Cervical cancers occur primarily at this cervical transformation zone. In the past decade, HPV has been identified as a necessary causal agent to the development of cervical neoplasia and can be detected in 99.7% of cervical cancers (Schiffman et al., 2011). The most common histologic types of cervical cancer are squamous cell (69% of cervical cancers) and adenocarcinoma (25%). Cervical cancer generally arises from acute infection with high-risk HPV carcinogenic subtypes, followed by viral persistence in the squamocolumnar junction and then invasion.
Early Disease
Preinvasive disease is usually detected during routine screening from cervical cytology. Patients with early invasive disease may also be asymptomatic.
![]() CLINICAL WARNING:
CLINICAL WARNING:
The first symptom of invasive cervical cancer is usually abnormal bleeding, which may be postcoital, irregular, or heavy. This may be associated with clear, watery, mucoid, purulent, or foul-smelling vaginal discharge. Initial symptoms may be clinically mistaken for cervicitis or vaginitis. Pelvic pain may result from invasion of the disease or from coexistent pelvic inflammatory disease. Asymptomatic women may be diagnosed when a visible lesion is discovered upon pelvic examination.
The triad of sciatic pain, leg edema, and hydronephrosis is almost always associated with extensive pelvic involvement by the tumor. Hydronephrosis may cause flank pain and may be associated with pyelonephritis.
![]() CLINICAL WARNING:
CLINICAL WARNING:
Patients with very advanced tumors may present with pelvic or low-back pain that radiates along the posterior side of lower extremities. Bowel or urinary complaints especially pressure, hematuria, hematochezia, or presence of stool in the vagina can also suggest advanced disease. Constipation may be a consequence of external compression of the rectum by the tumor.
Epidemiology
The ACS estimates that there are 12,000 new cases of invasive cervical cancer in the United States per year. Approximately 4,000 patients are expected to die of cervical cancer; this represents approximately 2% of all cancer deaths in women and 18% of all gynecologic cancers. Cervical cancer is the third most common gynecologic cancer in the United States (Siegel, Ma, Zou, & Jemal, 2014).
Squamous cell carcinoma (SCC) of the cervix follows a pattern of sexually transmitted disease. The International Agency for Research on Cancer (IARC) has identified 12 high-risk carcinogenic HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59 (Schiffman et al., 2011). Of these HPV subtypes, HPV 16 and 18 account for 70% of all cervical cancers. HPV 16 is most carcinogenic in terms of numbers of cases of SCC and cervical intraepithelial neoplasia grade 3 (CIN3). HPV 18 is more frequently seen in cases of adenocarcinoma. Research has revealed the distribution of HPV subtypes for SCC: HPV 16 (59% of cases); HPV 18 (13%); HPV 58 (5%); HPV 33 (5%); and HPV 45 (4%).
Adenocarcinoma is associated with HPV 16 (36%); HPV 18 (37%); HPV 45 (5%); HPV 31(2%); and HPV 33 (2%) (Li, Franceschi, Howell-Jones, Snijders, & Clifford, 2011).
HPV is the most common sexually transmitted infection in the United States. More than 40% of American women will develop genital HPV infection (Hariri et al., 2011). However, most high-risk HPV infections do not cause cancer and asymptomatically disappear within 1 to 2 years. HPV infection slowly progresses to cervical cancer with extremely few rapid-onset cases of cervical cancer occurring before the age of 25 years; most cancer diagnoses are made after 40 years of age (ACS, 2014; Castellsagué, 2008). HPV is not the exclusive cause of cervical cancer and there are rare HPV-negative cervical cancers. Other possible causal cofactors might act by either diminishing immunity or potentiating HPV infection. These include smoking, immunodeficiency, high parity, multiple sexual partners, oral contraceptive (OC) use, poor oral hygiene, chronic inflammation, and coinfection with other infectious agents (especially chlymadia, HIV, and herpes simplex virus (HSV)-2 infection; Barakat, Markman, & Randall, 2009; Castellsagué, 2008; Schiffman et al., 2011). In the United States, the incidence of cervical cancer is higher among women of certain racial and ethnic groups: Whites (incidence: 7.7/100,000 and mortality: 2.2/100,000); Hispanics/Latinos (12.5/100,000 and 3.1/100,000); African Americans (10.7/100,000 and 4.4/100,000); and American Indians and Alaska Natives (9.7/100,000 and 3.4/100,000; Siegel et al., 2014).
Globally cervical cancer continues to be the second leading cause of cancer deaths for women in less-developed countries. The highest incidence rates, more than five times the rates in the United States and Canada, were reported from East Africa, Central America, and the Pacific Islands (Barakat et al., 2009).
Diagnostic Criteria
Cervical changes are classified using the Bethesda system in the United States that incorporates a view of cervical carcinogenesis due to HPV infection (Solomon & Nayar, 2004). Liquid-based cytology techniques create more uniform slides and computer-assisted cytology evaluation systems have been adopted to achieve more rapid reporting and resulting. Definitive diagnosis of cervical cancer is based on histology obtained through cervical biopsy.
History and Physical Examination
All patients with cervical cancer should be evaluated with a careful history and physical examination, with particular attention to inspection and palpation of pelvic organs with bimanual and rectovaginal examinations. Attention to the psychosocial and cultural concerns of the patient is very important. The provider must consider the difficulties of this moment and should be comfortable and knowledgeable enough to encourage patients to express and discuss their concerns.
Diagnostic Studies
PAP SMEAR
Annual Pap smears are recommended for high-risk patients only. High-risk patients include patients who are cigarette smokers, are infected with high-risk HPV strains, have multiple-sexual partners, and those with prior abnormal Pap smear or cervical dysplasia. Women younger than age 21 years should no longer be screened with HPV testing or Pap smears. The false-negative rate of the Pap smear is about 10% to 20% in women with invasive cancer. The sensitivity of the test may be improved by proper sampling of the squamocolumnar junction and the endocervical canal. Smears without endocervical cells or metaplastic cells are inadequate and must be repeated. Because adenocarcinoma in situ (AIS) originates near or above the transformation zone, it may not be sampled by a conventional smear. Detection of high endocervical lesions might be improved with the use of a cytobrush. Extensive evidence from randomized clinical trials exists that HPV DNA screening is more sensitive than Pap smear screening alone for detecting precancerous cervical lesions (Ronco et al., 2010; Schiffman et al., 2011). A negative HPV test provides long-term risk stratification: 5 to 10 years of reassurance (i.e., a high negative predictive value) of not developing invasive cancer among HPV DNA–negative women. Presently, best practices suggest HPV testing in conjunction with Pap smear for woman aged 30 to 65 years (Schiffman et al., 2011).
HPV testing for screening of cervical cancer in women younger than 30 years is not recommended. Cytology every 3 years addresses screening for cervical cancer in women aged 21 to 29 years. Among women who are HPV positive but cytology negative about 60% become negative within 6 months (Apgar, Kittendorf, Bettcher, Wong, & Kaufman, 2009). However women aged 30 years or older have a greater risk of developing CIN3 within 10 years than their younger counterparts; 21% versus 14%, respectively. Women older than 30 years of age with negative cytology and negative HPV tests have a long-term probability of absence of cervical cancer. Therefore, in women who have had a hysterectomy or older than 65 years of age and without a history of severe diagnosis of CIN2 or higher, screening for cervical cancer may be discontinued.
Another option for women with high risk of HPV testing younger than 30 years of age is reflex HPV testing. Reflex HPV testing refers to a process in which the HPV test is only performed if the Pap smear result is abnormal. If the Pap smear result is normal, it is not performed. It is recommended that women with negative cytology and positive HPV testing may have repeat testing in 12 months. If HPV high-risk infection persists, colposcopy is indicated even if cytology is negative. Screening by HPV testing alone is currently not recommended at any age.
Clinical Pearls
 Pap smears without endocervical cells or metaplastic cells are inadequate and must be repeated.
Pap smears without endocervical cells or metaplastic cells are inadequate and must be repeated.
![]() CLINICAL WARNING:
CLINICAL WARNING:
Regardless of hysterectomy, women older than 65 years, with a past history of CIN2 or a more severe diagnosis, should continue routine screening for cervical cancer for at least 20 years.
COLPOSCOPIC EXAMINATION
Patients with abnormal cytology and no gross lesion must be evaluated by colposcopy and directed biopsy. In 2006, the American Society for Colposcopy and Cervical Pathology (ASCCP) convened a conference to create guidelines for management of women with abnormal cervical cancer screening tests and CIN based on the best available evidence (Massad et al., 2013). It should be noted that colposcopy is diagnostic for women with abnormal cervical cytologic results but is not an effective screening tool. The efficacy of colposcopy depends upon the experience and training of the colposcopist. Therefore, the primary care clinician should refer all suspicious lesions to a skilled colposcopist.
ENDOCERVICAL CURETTAGE
If the entire squamocolumnar junction cannot be visualized on colposcopy in a patient with an atypical Pap smear, endocervical curettage (ECC) is indicated. ECC is also indicated in patients with atypical squamous columnar lesions (ASCH); high-grade squamous intraepithelial lesions (HSIL); atypical glandular columnar cells (AGC; AIS; ASC-US/LSIL) but no visible lesion (Solomon et al., 2007).
CONE BIOPSY
Cervical cone biopsy is potentially therapeutic when used to diagnose occult endocervical lesions and is an important step in the diagnosis of microinvasive carcinoma of the cervix. Excisional treatment of the carcinoma can be performed using a scalpel, laser, or electrosurgery (i.e., cold knife coniation, loop electrosurgical excision procedure [LEEP], also called large loop excision of the transformation zone [LLETZ]). There is presently no evidence that one technique is significantly better than another. It is indicated in the following cases.
 When the squamocolumnar junction is poorly visualized on colposcopy and a high-grade lesion is suspected
When the squamocolumnar junction is poorly visualized on colposcopy and a high-grade lesion is suspected
 When a high-grade dysplastic epithelium extends into the endocervical canal
When a high-grade dysplastic epithelium extends into the endocervical canal
 When cytology results suggest carcinoma in situ
When cytology results suggest carcinoma in situ
 When ECC shows HSIL
When ECC shows HSIL
 When there is suspicion of AIS (Martin-Hirsch, Paraskevaidis, Bryant, & Dickinson, 2013)
When there is suspicion of AIS (Martin-Hirsch, Paraskevaidis, Bryant, & Dickinson, 2013)
Cone biopsy should be performed in pregnant women only when there is a strong suspicion of invasive cancer.
LABORATORY STUDIES
Standard laboratory studies should include a complete blood count (CBC) and renal and liver function tests in all patients with invasive cervical cancer. Since treatment of choice involves hysterectomy, patient’s comorbid conditions should be assessed, and preoperative coagulation profiles should be drawn.
Primary care clinicians should be cautioned regarding the use of available tumor markers. A number of serum markers have been investigated for use in assessing prognosis, monitoring response to therapy, and detecting recurrence. The most commonly used are serum SCC antigen, tissue polypeptide antigen, CEA, CA-125, and CYFRA 21–2. No significant research exists to support their use in primary care and they are not recommended as routine diagnostic tests. CA-125 levels are elevated in only 13% to 21% of women with cervical squamous cell cancer, but may be a better tumor marker for those with adenocarcinoma (Amendola et al., 2005).
RADIOLOGIC STUDIES
All patients with invasive cervical cancer should have a chest radiograph to rule out metastatic disease to the lungs, and imaging including CT scan or MRI obstruction by the tumor.
Treatment Options, Expected Outcomes, and Comprehensive Management
Several factors may influence treatment options, including tumor size, stage, and histology; evidence of lymph node involvement; risk factors for surgery or radiation; and patient preference. The International Federation of Gynecology and Obstetrics (FIGO) has defined the most widely accepted staging system for carcinomas of the cervix; it was last updated in 2009 (Mutch, 2009). As a rule, intraepithelial lesions are treated with superficial ablative techniques such as cryosurgery, laser therapy, or loop excision. These are all outpatient procedures that maintain fertility and carry a low-recurrence rate; progression to invasion is rare. Lifelong surveillance of these patients is necessary, however, to detect early signs of recurrence.
Microinvasive cancers invading less than 3 mm (stage IA1) are managed with conservative surgery, such as excisional conization or extrafascial hysterectomy. Total or vaginal hysterectomy is the standard treatment for this stage of disease. However for those who wish to maintain fertility, conization is the choice. Conization is performed with a cold knife or carbon dioxide laser under general or spinal anesthesia. Complications occur in 2% to 12% of patients and include sepsis, hemorrhage, infertility, stenosis, and cervical incompetence.
Early invasive cancers (Stages IA2 and IB1 and some small Stage IIA tumors) that are confined to the cervix, uterus, upper third of the vagina, are nonbulky (4 cm or less), and have no lymph node metastases are appropriate candidates for radical hysterectomy. Cancers with local extension (the low two-thirds of the vagina, parametrium, or bladder) that are bulky (stage IB2, IIA2 to IVA) or with lymph node metastases are generally treated with chemoradiation. Assessment of lymph nodes helps to design the radiation fields (i.e., pelvic radiation vs. extended field radiation). The prognosis is influenced by tumor characteristics such as bulk and diameter, lymph node metastasis, histology, and hemoglobin level (Mutch, 2009). Patients with widely metastatic disease (e.g., carcinomatosis or lung metastases; Stage IVB) are typically treated with full-dose chemotherapy alone. Palliative radiation therapy may be used to reduce vaginal bleeding or pain. Advanced care planning conversations with these women is essential in primary care to reduce pain, suffering, and loss of dignity at end of life (see Chapter 5).
SCREENING
The long preinvasive stage of cervical cancer, the relatively high prevalence of the disease in unscreened populations, and the sensitivity of cytologic screening all have made cervical carcinoma an ideal target for cancer screening. In the United States, screening with cervical cytology, as well as performing frequent pelvic examinations, has led to a decrease in the incidence rate from cervical cancer of more than 70% since the 1960s (Chelmow, Waxman, Cain, & Lawrence, 2012). Cervical cancer, once one of the most frequent cause of cancer deaths in women, now ranks 14th for cancer deaths (Siegel et al., 2014).
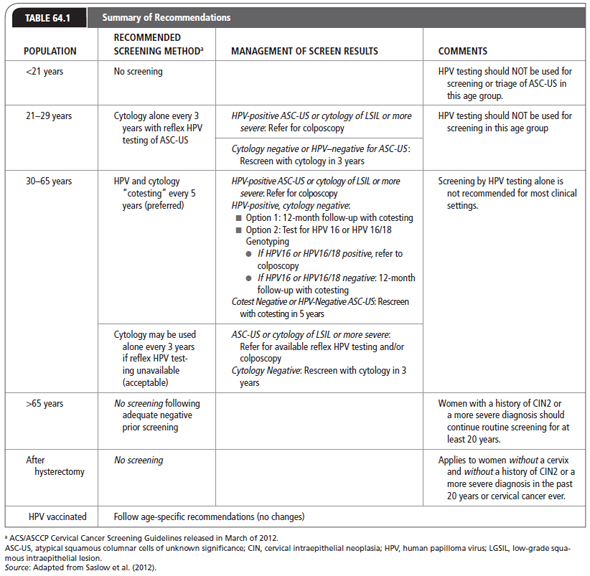
Cervical cancer is also one of the most successfully controlled cancers in developed countries due to advances in the use of the Papanicolaou test as a screen. Women who have precancerous lesions detected through Pap tests have a nearly 100% 5-year survival rate. In contrast, 60% to 80% of women with advanced cervical cancer have not had a Pap test in the 5 years preceding diagnosis of cancer (ACS, 2012). In March 2012, the ACS, the ASCCP, and the American Society for Clinical Pathology (ASCP) released screening guidelines for cervical cancer screening to incorporate best practices in utilizing new HPV testing technology (Massad et al., 2013; Saslow et al., 2012). The revised recommendations suggest screening for all women to begin at age 21 years; for women 21 to 29 years, cytology alone every 3 years is recommended; for women 30 to 65 years, cytology with HPV cotesting every 5 years is suggested; however, if HPV testing is unavailable, then cytology alone every 3 years should be continued until age 65 years. Women without history of CIN 2 or higher-cervical disease may discontinue screening at age 65 years or after hysterectomy with removal of the cervix (Saslow et al., 2012).
Patients who were diagnosed and treated for cervical cancer should be followed by the provider over their lifetime to maintain health. Periodic examinations will prevent or at least delay the recurrence of the disease and further complications. Table 64.1 summarizes recommendations for screening.
PREVENTION
As a mention of public health policy, the introduction of HPV vaccination in the new millennium has strengthened best practices of primary prevention of cervical cancer. There are presently two licensed HPV prophylactic vaccines—Gardasil (Merck) and Cervarix (GlaxoSmithKline) with research supported efficacy against new infection with HPV 16 and HPV 18, HPV 31, HPV 45, CIN2, and CIN3 lesions (Paavonen et al., 2009). Presently, the American Academy of Immunization Practices recommends HPV vaccination of boys and girls before the median age of first sexual intercourse. Herd immunity conferred by an effective multivalent HPV vaccine will be the best primary preventive strategy against cervical cancer. HPV vaccination will not help older women who are beyond the age of vaccination (>26 years of age) or those who already have persistent HPV infection.
![]() CLINICAL WARNING:
CLINICAL WARNING:
Patients with abnormal Pap smears should be followed closely by the primary care provider with periodic Pap smears, colposcopy, and biopsy in case of LSIL. When needed, referral should be made to a gynecologic oncology practice that performs cone biopsies and loop and laser procedures; surgical procedures such as hysterectomy in case of HSIL or invasive carcinoma come under the venue of such a practice. Primary care clinicians can improve prevention through active education and use of HPV vaccination.
Any abnormal bleeding is suspicious and must be investigated.
 CARCINOMA OF THE VAGINA
CARCINOMA OF THE VAGINA
Anatomy, Physiology, and Pathology
The vagina extends from the vulva to the uterus. It is situated in the pelvic cavity behind the bladder and in front of the rectum. It is about 2 inches along its anterior wall and 3 inches along its posterior wall. The vaginal epithelium is of the squamous type.
Carcinoma of the vagina compromises 3% of all female gynecologic malignancies. SCCs account for 80% to 90% of primary vaginal malignancies, adenocarcinomas for 10%, vaginal melanomas for 3%, and vaginal sarcomas for another 3% (Young, Higgins, Yuh, & Mayr, 2014).
Epidemiology
PRIMARY VAGINAL CARCINOMA
In 2014, an estimated 3,000 American women will develop SCC of the vagina and 900 will die from the illness (Siegel et al., 2014). Primary carcinomas of the vagina are rare and represent 2% to 3% of gynecologic malignancies. Unlike other gynecologic malignancies, vaginal carcinoma is not related to reproduction or use of exogenous hormones. Due to the rarity of SCC of the vagina, there is a paucity of clinical evidence demonstrating risk factors and etiology of the disease (Alonso et al., 2012; Di Donato et al., 2012). Epidemiologic studies have suggested associations with low socioeconomic status, histories of genital warts or other genital irritations, prior abnormal Pap smears, multiple sexual partners, early ages at first intercourse, and current cigarette smoking (ACS, 2013; Di Donato et al., 2012). Moreover, there is an increased risk of vaginal cancer with HSV-2 infection, HIV infection, use of pessaries, vaginal douching, and chronic vaginitis (Narayanan & Sohaib, 2013). The highest risk of development of vaginal carcinoma has been associated with prior hysterectomies secondary to cervival cancer most likely mediated by HPV infection (Brown, 2013). Therefore, the development of vaginal carcinoma is likely associated with the same risks factors as the development of cervical neoplasia.
The close correlation of HPV infection and vaginal carcinoma has been supported by 30% of women with vaginal carcinoma reporting prior surgical excision of cervical carcinoma (Patrelli et al., 2011). Recent clinical studies, have demonstrated HPV infection through findings of HPV DNA in vaginal cancer tissue (Narayanan & Sohaib, 2013). HPV was present in 60% of invasive carcinoma and 80% of in situ vaginal SCCs. The presence of HPV 16 increased the risk of development of in situ vaginal carcinoma by up to 13 times (Narayanan & Sohaib, 2013).
Histologically, most primary vaginal neoplasms occur in the posterior wall of the upper third of the vagina and are squamous cell (83%) but adenocarcinoma, sarcoma, and melanoma account for 9%, 3%, and 3%, respectively. Although primary vaginal carcinoma is rare, vaginal metastasis is common either by direct extension (i.e., cervix, vulva, endometrium, rectum, or bladder) or by lymphatic spread (i.e., breast, ovary, or kidney). The incidence of vaginal carcinoma is higher for Blacks (1.1 per 100,000 for blacks vs. 0.7 per 100,000 for Whites), but the reasons for the discrepancy are unknown (Barakat et al., 2009). However the 5-year survival rate for all ethnicities is 50% (Siegel et al., 2014). Primary invasive carcinoma of the vagina is predominantly a disease of elderly women, with 60% to 70% of cases presenting in women older than age 60 years (except for clear-cell carcinomas, which are associated with maternal exposure to diethylstilbestrol [DES]; Barakat et al., 2009).
INTRAEPITHELIAL VAGINAL DYSPLASIA OF GLANDULAR ORIGIN, OR ATYPICAL VAGINAL ADENOSIS
Intraepithelial vaginal dysplasia of glandular origin, or atypical vaginal adenosis, is histologically clear-cell adenocarcinoma. In 1971, the development of clear-cell adenocarcinomas in young women (aged 15–22 years) was correlated with DES exposure in utero in New York State and at the Mayo Clinic (Troisi et al., 2007). The majority of patients with vaginal or cervical adenocarcinomas related to DES are diagnosed prior to age 25 years, with the incidence after this age decreasing by 80% (Troisi et al., 2007). The risk of developing clear-cell adenocarcinoma through age 34 years, for women exposed to DES is 1 in 1,000 (Troisi et al., 2007). The highest risk occurs in women who were exposed before 12 weeks’ gestation.
VAGINAL INTRAEPITHELIAL NEOPLASIA
Vaginal intraepithelial neoplasia (VAIN) is a precursor to secondary development of carcinoma of the vagina. VAIN is defined by the presence of squamous cell atypia without invasion. VAIN may precede the development of carcinoma of the vagina and is predominately seen in women older than 60 years of age. VAIN occurs in the upper-vaginal cuff and occurs often in concomitant cervical and vulvar HPV infection. VAIN is classified according to the depth of epithelial involvement in a similar manner as CIN. In 2012, as part of a standardization project for HPV-associated lesions, the College of American Pathologists and the ASCCP revised terminology by which VAIN is reported using a two-tiered nomenclature: LSIL for low-grade disease (VAIN 1) and HSIL for high-grade disease (VAIN 2/3; Darragh et al., 2012). The incidence of VAIN or vaginal carcinoma in situ is 0.1 to 0.2 per 100,000 women (Young et al., 2014).
Most patients with vaginal neoplasms are asymptomatic at diagnosis. The carcinoma is usually diagnosed by biopsy during an investigation of an abnormal Pap smear. The FIGO categories are used for staging vaginal cancers (Mutch, 2009).
The FIGO system classifies vaginal carcinoma in Stages 0 through IV depending on the extent of the tumor (T), whether the cancer has spread to lymph nodes (N), and whether it has spread to distant sites (M for metastasis). The use of PAP smear screening for vaginal carcinoma is not recommended due to the rarity of the disease.
![]() CLINICAL WARNING:
CLINICAL WARNING:
About 65% to 80% of patients with invasive cancer present with abnormal vaginal bleeding, frequently postcoital or postmenopausal. Vaginal discharge, a palpable mass, dyspareunia, and perineal or pelvic pain are other complaints that may be expressed at presentation (Narayanan & Sohaib, 2013). Advanced disease may present as hematuria or urinary retention when located anteriorly or as constipation, melena, or tenesmus when located posteriorly near the rectum.
History and Physical Examination
The workup should include a careful examination of the cervix and vagina, including a bimanual examination. A careful history and assessment of the woman’s psychosocial status and cultural needs should be included.
Digital palpation during the pelvic examination should be used to assess for thickening or irregularity of the vaginal wall. The use of acetic acid application during the examination may identify sharply demarcated lesions that may appear as white raised or flat lesions. Strong clinical suspicions of vaginal carcinoma should be referred for thorough colposcopic assessment of the entire vagina. In the postmenopausal patient, a few weeks of topical estrogen (ER) treatment may accentuate visualization and improve detection of VAIN. The presence of a markedly irregular surface or severe vascular abnormalities with unusual branching suggests an invasive process, which warrants urgent referral for excisional biopsy. Schiller’s or Lugol’s iodine solution can be used to detect lesions and confirm boundaries prior to excision by a trained colposcopist.
Diagnostic Studies
All patients should have chest and skeletal radiography, CBC, and biochemical profile. Cystoscopy and ureteroscopy are strongly recommended for patients with large tumors and tumors involving the anterior wall of the vagina. Anoscopy or sigmoidoscopy is recommended for lesions involving the posterior wall of the vagina.
![]() CLINICAL WARNING:
CLINICAL WARNING:
Vaginal biopsy and colposcopy are indicated only when abnormalities are noted on physical examination. Routine use of computed axial tomography (CAT) scan or PET scan is not recommended and should only be performed if recurrent disease is suspected.
Treatment Options, Expected Outcomes, and Comprehensive Management
There is no current consensus or standard chemotherapy treatment of vaginal carcinoma. Early stage tumors are often curable with local therapies (i.e., surgical excision, ablation, topical therapy, or radiation), but there is no curative standard treatment of proven efficacy for metastatic disease.
Several treatments factors must be considered including the patients’ psychosexual desires to maintain sexual relations and a functioning vagina. The anatomy of the bladder, urethra, and rectum prevents administration of high-dose radiation and may not permit large surgical excision of lesions.
 Stage 0: Management ranges from no treatment for VAIN Type 1, to laser ablation for VAIN 2/3, to surgery. Lesions <2 cm are usually treated by surveillance.
Stage 0: Management ranges from no treatment for VAIN Type 1, to laser ablation for VAIN 2/3, to surgery. Lesions <2 cm are usually treated by surveillance.
 Stage I: Radiotherapy is the treatment of choice. Five-year survival rates are 83% to 100%.
Stage I: Radiotherapy is the treatment of choice. Five-year survival rates are 83% to 100%.
 Stage II: Radiotherapy or radical surgery is indicated. Five-year survival rates are 68% to 95%
Stage II: Radiotherapy or radical surgery is indicated. Five-year survival rates are 68% to 95%
 Stages III and IV: Radiotherapy is indicated. Survival rates are 44% to 65% for Stage III and 13% to 30% for Stage IV (Tran et al., 2007).
Stages III and IV: Radiotherapy is indicated. Survival rates are 44% to 65% for Stage III and 13% to 30% for Stage IV (Tran et al., 2007).
Early-stage disease and low-risk disease should be evaluated posttreatment every 6 months for the first 2 years and then annually. For patients with high-risk disease, patients should be reexamined every 3 months for the first 2 years and then every 6 months thereafter until the patient reaches 5 years postsurvival.
 CANCER OF THE VULVA
CANCER OF THE VULVA
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


