GASTROINTESTINAL EMERGENCIES
ERIC A. RUSSELL, MD, BRUNO P. CHUMPITAZI, MD, MPH, AND CORRIE E. CHUMPITAZI, MD
GOALS OF EMERGENCY CARE
Abdominal complaints are among the most common reasons for a child to present to the emergency department. While the majority of these complaints are not life-threatening, the emergency provider must be able to recognize the most serious diagnoses and initiate appropriate therapy. The goal for the ED physician is to identify and stabilize gastrointestinal (GI) emergencies early in their course. This includes the recognition of hemodynamic instability or impending instability secondary to infection, bleeding, organ dysfunction, or ischemia. This can be aided by the identification of patients at high risk for life-threatening emergencies such as those with a history of abdominal surgery, organ transplantation, biliary manipulation, immunodeficiency, or primary hepatic, pancreatic, oncologic, or renal disease.
RELATED CHAPTERS
Signs and Symptoms
• Abdominal Distension: Chapter 7
• Gastrointestinal Bleeding: Chapter 28
• Jaundice: Conjugated Hyperbilirubinemia: Chapter 39
• Jaundice: Unconjugated Hyperbilirubinemia: Chapter 40
Clinical Pathways
• Abdominal Pain in Postpubertal Girls: Chapter 82
• Appendicitis (Suspected): Chapter 83
Medical, Surgical, and Trauma Emergencies
• Infectious Disease Emergencies: Chapter 102
• Oncologic Emergencies: Chapter 106
• Renal and Electrolyte Emergencies: Chapter 108
• Abdominal Trauma: Chapter 111
• Abdominal Emergencies: Chapter 124
• Thoracic Emergencies: Chapter 132
KEY POINTS
 Abdominal pain may be a presenting sign of systemic disease.
Abdominal pain may be a presenting sign of systemic disease.
 Brisk bleeding is uncommon but can rapidly become life-threatening.
Brisk bleeding is uncommon but can rapidly become life-threatening.
 GI bleeding may be a sign of bowel ischemia.
GI bleeding may be a sign of bowel ischemia.
 Previous hepatic or biliary surgery places a patient at high risk for cholecystitis and ascending cholangitis.
Previous hepatic or biliary surgery places a patient at high risk for cholecystitis and ascending cholangitis.
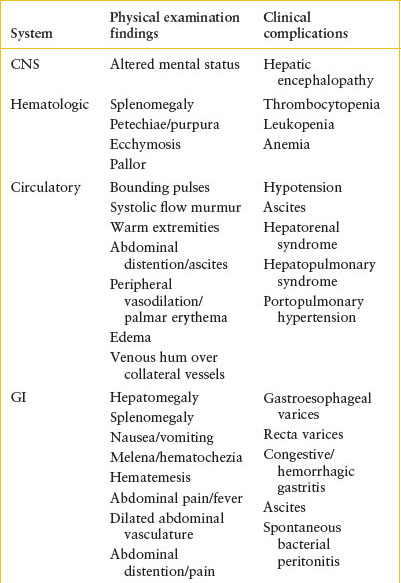
 Hepatic encephalopathy is not universally seen in patients with acute liver failure (ALF), but when identified, may be a life-threatening finding of ALF.
Hepatic encephalopathy is not universally seen in patients with acute liver failure (ALF), but when identified, may be a life-threatening finding of ALF.
GASTROINTESTINAL BLEEDING
Goals of Treatment
GI bleeding is a common and occasionally life-threatening condition in infants and children. An orderly approach to this problem is essential (see Chapter 28 Gastrointestinal Bleeding). Significant GI bleeding places a patient at risk of circulatory collapse. The goal for the ED physician is to address life-threatening GI bleeding by stopping the ongoing losses and replacing intravascular volume. Addressing ongoing bleeding will require a team of professionals, including the emergency physician, surgeon, and/or gastroenterologist. Addressing potential circulatory compromise achieves two principal objectives: Oxygen carrying capacity is improved through administration of blood products and the perfusion pressure to vital organs is preserved via blood product and intravenous (IV) fluid administration.
The vast majority of patients with either upper or lower GI bleeding will not have experienced significant blood loss. These patients can be managed successfully with judicious laboratory investigation, supportive care, and follow-up with a primary care provider or an appropriate subspecialist.
Upper Gastrointestinal Bleeding
Esophageal Varices
Goals of Treatment. The initial goals of therapy of suspected variceal hemorrhage are identical to those of massive upper GI bleeding from any source. Volume resuscitation to maintain adequate perfusion and oxygen carrying capacity is necessary, but overexpansion of the intravascular volume should be avoided because it may contribute to rebleeding. Patients with actively bleeding esophageal varices (EV) may also have liver dysfunction and, as a result, early therapy should also correct existing coagulopathies.
Current Evidence. Upper GI bleeding from EV is a major cause of morbidity and mortality in patients with underlying liver disease and portal hypertension. Causes of EV can be seen in Table 99.1. Varices that develop as a result of portal hypertension are a type of portal-systemic collateral, which develop secondary to the abnormally elevated pressure within the portal system and can form in any area where veins draining the portal venous system are in close approximation to veins draining into the caval system (i.e., submucosa of the esophagus, submucosa of the rectum, and anterior abdominal wall). Patients with EV often have underlying portal hypertension, and their varices may develop over a few months or after many years. Patients with portal hypertension are also at risk of GI bleeding from congestive or hemorrhagic gastritis.
CLINICAL PEARLS AND PITFALLS
• Patients with varices are likely to have portal hypertension, and therefore are at risk for ascites, spontaneous bacterial peritonitis, bleeding, and splenomegaly with associated thrombocytopenia and leukopenia.
• Overexpansion of intravascular volume may contribute to rebleeding.
• Patients with portal hypertension are also at risk for bleeding from congestive gastritis.
• Coagulation abnormalities in the setting of active bleeding should be managed aggressively with IV vitamin K, fresh frozen plasma (FFP), and platelets. Coagulation abnormalities without active bleeding do not require FFP or platelets.
• Prophylactic antibiotics are part of initial pharmacologic management. Bleeding varices may be the initial sign of sepsis in patients with liver disease.
• Octreotide is part of initial pharmacologic management with severe active bleeding due to portal hypertension.
EV are very common in patients with certain types of high-risk underlying liver disease, particularly biliary atresia (BA) and portal vein thrombosis where EV have been reported to be present in as many as 70% of patients. Patients are at increased risk for EV if they have splenomegaly, thrombocytopenia, or hypoalbuminemia. In addition to primary liver disease, patients with congestive heart failure are known to be at high risk for EV. These factors should be taken into account when evaluating a patient with a history of an upper GI bleed or when counseling families for their risk of upper GI bleed.
TABLE 99.1
CAUSES OF PORTAL HYPERTENSION AND ESOPHAGEAL VARICES

Clinical Considerations
Clinical Recognition. Patients with EV may have occult bleeding, but more commonly, the bleeding is brisk. Patients will have hematemesis, hematochezia, and/or melena. The possibility of bleeding EV should be considered in any patient with a history of jaundice (beyond the newborn period), hepatitis, ascites, chronic right-sided heart failure, portal vein thrombosis, pulmonary hypertension, omphalitis, umbilical vein catheterization, or one of the hepatic parenchymal diseases noted in Table 99.1.
Triage Considerations. While it is common that bleeding will have stopped prior to arrival in the ED, patients with EV have the potential for significant blood loss. Close attention should be given to tachycardia as an early indicator of hemodynamic compromise and patients should be triaged accordingly. Patients with significant upper GI bleeding may also be at risk for airway compromise.
Clinical Assessment. One should have a high suspicion of EV in any patient presenting with an upper GI bleed and any of the risk factors listed above. One can also evaluate for the stigmata of portal hypertension, such as jaundice, ascites, rectal hemorrhoids, and hepatosplenomegaly (Table 99.2). Other signs or symptoms of right-sided heart failure would also place a patient at higher risk. Given the risk for sudden and life-threatening bleeding, assessing this risk is essential.
In patients with severe upper GI bleeding from EV, two large-bore IVs should be started immediately (Fig. 99.1). An nasogastric (NG) tube should be placed to evaluate for ongoing bleeding and to remove blood from the stomach, which may act as an irritant and potentially worsen hepatic encephalopathy. Variceal bleeding is not a contraindication for passing an NG tube. Immediate laboratory studies should include type and crossmatch, complete blood cell (CBC) count, platelet count, prothrombin time (PT), and partial thromboplastin time (PTT). Additional laboratory studies may be indicated on the basis of the differential diagnosis of the most likely cause of the patient’s bleeding. Arterial blood gases should also be followed when severe blood loss is associated with shock. The hematocrit is an unreliable initial index of acute blood loss because it may be normal or only slightly decreased and not accurately reflect the actual value in a rapid bleed.
TABLE 99.2
SIGNS AND SYMPTOMS OF PORTAL HYPERTENSION
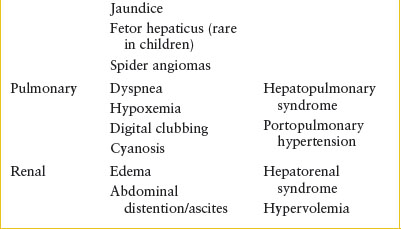
Transfusion of blood products may be necessary to maintain adequate end-organ perfusion with severe GI bleeding. The volume of blood products that should be administered as well as the timing of those transfusions is controversial. Current recommendations generally target a hemoglobin transfusion goal of 7 to 8 g/dL. Excessive blood administration, especially in the setting of non–life-threatening bleeding, should be avoided, as it is known to contribute to rebleeding. Coagulation abnormalities should be managed aggressively only if there is active bleeding, as transfusion of blood products may lead to volume overload. In patients who are not actively bleeding, the ED physician should not attempt to correct a coagulopathy as a patient’s PT and INR can be very difficult to correct and are not reliable indicators of bleeding risk in those with underlying liver disease. It is also important to note that bleeding varices may be the initial sign of sepsis in patients who have cirrhosis. Prophylactic broad-spectrum antibiotics, after appropriate cultures are obtained, are recommended in the setting of a significant esophageal bleed.
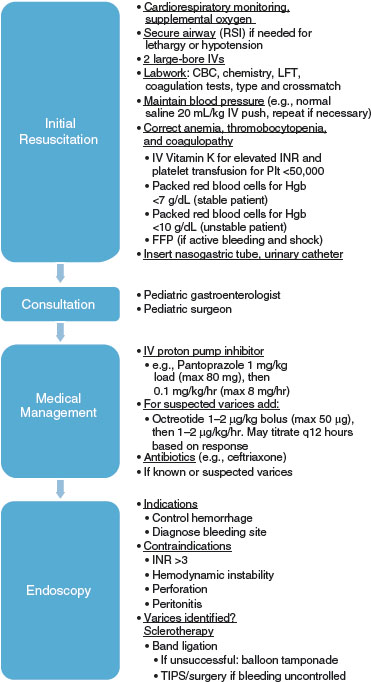
FIGURE 99.1 Emergency management of severe UGI bleeding. The management involves a multidisciplinary approach from resuscitation, to consultation, to medications, to endoscopy if indicated.
Therapies for acute variceal hemorrhage include both pharmacologic and endoscopic therapy. Multiple pharmacologic agents have been studied, including vasopressin, somatostatin, and octreotide. Octreotide has been found to be the most effective with the best side effect profile and is the preferred initial pharmacotherapy. It may be given at a dose of 1 µg/kg followed by 1 to 5 µg/kg/hr continuous infusion.
Emergency flexible esophagogastroduodenoscopy should be arranged if the patient remains hemodynamically unstable after initial pharmacologic therapies. Endoscopic techniques used for acute management of variceal bleeding include endoscopic variceal sclerotherapy and band ligation. Ideally, endoscopy will be performed within 24 hours (Fig. 99.1).
Peptic Ulcer Disease
Goals of Treatment
Peptic ulcer disease (PUD) often presents with nonspecific symptoms of abdominal pain, dyspepsia, and heartburn to varying degrees of severity. In rare cases, children present with GI hemorrhage, GI perforation, or gastric outlet obstruction. The primary goal of ED management is to identify the rare child with a significant complication from PUD that requires further stabilization and management (see Chapter 28 Gastrointestinal Bleeding). In the majority of cases, once PUD is identified as a potential cause of abdominal pain, it is appropriate to initiate outpatient diagnostic testing, start a gastric antisecretory regimen, and ensure close follow-up.
CLINICAL PEARLS AND PITFALLS
• Stress-related ulcers are common causes of PUD in early infancy.
• The most common causes of PUD in older children are Helicobacter pylori and nonsteroidal anti-inflammatory drug (NSAID) usage.
• Gastric outlet obstruction from PUD should be considered in the child with chronic nonspecific abdominal symptoms and frequent nonbilious emesis of both liquids and solids at time of presentation.
• Proton pump inhibitors (PPIs), if discontinued abruptly, may result in rebound hypersecretion and exacerbation of symptoms.
Clinical Evidence
While the term PUD describes a group of disorders which involves changes to the mucosal lining of the upper GI tract (esophagus, stomach, and duodenum), this section will focus on diseases of the stomach and duodenum. PUD has various levels of severity with the most common causes of ulcers in stomach and duodenum being H. pylori infection followed by NSAIDs. Less common etiologies include stress-induced gastropathy, portal gastropathy, caustic ingestion, inflammatory bowel disease (IBD), and eosinophilic gastritis.
Ulcer disease occurs when there is an imbalance between cytotoxic factors, (e.g., acid, pepsin, NSAIDs, H. pylori) and cytoprotective factors, including the secretion of mucus and bicarbonate by superficial epithelial and mucous cells in the upper GI tract. Local blood flow, delayed gastric emptying, duodenal reflux, and other factors have been suggested as important factors in the development of gastric ulceration, but the exact pathogenesis of the condition remains unclear.
The role of the bacterium H. pylori in the etiology of ulcer disease in children has been investigated. H. pylori produces a localized inflammatory reaction that contributes to epithelial damage either by direct toxic effect or via immunopathologic means. H. pylori infection usually occurs in childhood with earlier acquisition and higher prevalence noted in developing countries. There are higher prevalence rates among family members and institutionalized populations, suggesting person-to-person transmission via either an oral route or a fecal-to-oral route. A family history of ulcer disease is typically present in 50% or more of children with duodenal ulcers.
Clinical Considerations
Clinical Recognition. Symptoms of PUD vary with the patient’s age. Stress ulcers account for 80% of peptic disease in early infancy, and often present as medical emergencies. Infants may present either with nonspecific feeding difficulties and vomiting, or with upper GI bleeding or perforation. Nonspecific signs and symptoms predominate among older infants and preschool-aged children, with boys and girls affected equally. Preschool-aged children often complain of poorly localized abdominal pain, vomiting, or GI hemorrhage, which can manifest as either hematemesis or melena. Among teenagers with ulcer disease, a male predominance is seen, with boys outnumbering girls nearly 4:1. Older children and adolescents generally present with abdominal pain, which is classically described as waxing and waning, sharp or gnawing, and localized to the epigastrium. It may awaken the child at night or in the early hours of the morning. Other historical clues include a family history of ulcer disease and the presence of predisposing factors such as smoking or regular use of NSAIDs.
Initial Assessment/H&P. History should focus on the presence of hematemesis and whether melanotic stools have been passed. Physical examination may reveal orthostasis, pallor, as well as abdominal tenderness, which is poorly localized in young children, but localized to the epigastrium or to the right of the midline in older children and adolescents. Stool should be tested for occult blood. The remainder of the physical examination should include an oral examination looking for dental enamel erosion, which would suggest chronic gastroesophageal reflux (GER) or recurrent emesis, and an examination of the lungs for wheezing, which also might suggest bronchospasm due to or exacerbated by reflux. Weight loss may be noted.
Management/Diagnostic Testing. A CBC and fecal occult blood test are good screening tests when one is considering the possibility of significant PUD. If physical examination findings reveal significant abdominal tenderness with guarding or rebound tenderness, plain radiographs of the abdomen including an upright view should be obtained to evaluate for perforation or secondary bowel obstruction. IV access should be obtained in all patients who have significant emesis, dehydration, weight loss, or concerning abdominal examination findings. An initial bolus of normal saline (20 mL per kg) should be given and vital signs monitored frequently, with additional boluses given as needed to achieve hemodynamic stability.
An upper GI series is not recommended for the routine evaluation of PUD given its relatively poor sensitivity. However, it may be useful in diagnosing children with gastric outlet obstruction secondary to PUD. Noninvasive H. pylori testing using either fecal antigen testing and/or C13 urea breath test should be undertaken in all patients for whom an obvious cause of secondary gastric or duodenal ulceration (e.g., NSAIDs, stress, sepsis, or burns) does not exist. Practice guidelines by the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) recommend initial diagnostic H. pylori testing with positive histopathology on EGD plus a positive rapid urease test or a positive culture. Tissue culturing of the organism may assist in the determination of appropriate antibiotic therapy, particularly in communities where antibiotic resistance is high. Serologic tests based on the detection of antibodies (IgG, IgA) against H. pylori in serum, whole blood, urine, and saliva are not reliable for use in the clinical setting.
A number of approaches are available for the treatment of PUD. Therapies can be categorized as those that neutralize acid (i.e., antacids), block acid secretion, are cytoprotective, or are anti-infective. Antacids are a low-cost, safe, primarily short-term means of treating PUD in children. They can be prescribed for patients of any age. Adverse effects of antacids are related to the metal ion present in the preparation: Magnesium-containing products may cause diarrhea, whereas aluminum-containing products may cause constipation. Some products are available as a combination of the two to minimize these effects. The usual dosage for children is 0.5 mL per kg, given 1 hour after eating and before going to the bed. Patients with food-related or nocturnal abdominal pain without associated signs of serious illness can be started on empirical therapy with antacids, and followed by a primary care physician.
H2-receptor antagonists (e.g., ranitidine or famotidine) are used to block acid secretion and treat ulcer disease. Alternatively PPIs (e.g., omeprazole or lansoprazole) can be used and are more effective at decreasing gastric acid secretion than H2-receptor antagonists. PPIs should be used in patients with anemia or who have moderate to severe PUD. Potential short-term adverse effects with PPI usage include headache, abdominal pain, diarrhea, nausea, and vomiting. When initiating therapy, follow-up is important, as is providing a sufficient quantity so that the child can take the medication until the outpatient visit has occurred.
Sucralfate (40 to 80 mg/kg/day by mouth every 6 hours) is an aluminum salt that insulates the gastric mucosa from further damage by acid, pepsin, or bile. It is recommended to be given on an empty stomach 1 hour before meals and at bedtime, and not given at the same time as other medications given its ability to bind them.
Current protocols for first-line therapy for H. pylori include a PPI plus two antibiotics (choosing two of the following: amoxicillin, clarithromycin, or metronidazole for 10 to 14 days). Compliance is an important consideration because it is a major determinant of the success of treatment. Antibiotic susceptibility testing for clarithromycin is recommended before initial clarithromycin-based triple therapy in areas/populations with a known high resistance rate (>20%) as this will adversely affect eradication rates.
Mallory–Weiss Tears/Prolapse Gastropathy
Mallory–Weiss tears are mucosal lacerations of the distal esophagus and proximal stomach induced by forceful retching. Patients typically present with a recent history of repeated vomiting prior to onset of the hematemesis and less frequently, with pain from the tear. While the amount of blood can vary, Mallory–Weiss tears usually are self-limited and do not require any medical or surgical intervention. Upper GI bleeding after retching or vomiting may also be due to prolapse gastropathy, when the stomach prolapses through the lower esophageal sphincter causing mucosal injury. Management is generally conservative with antiemetic therapy, PPI, and observation.
Lower GI Bleeding
Inflammatory Bowel Disease
Goals of Treatment. The goals of emergency therapy include initial supportive care and the identification of any IBD complication. These complications may include perforation, intra-abdominal or perirectal abscess, toxic megacolon, severe anemia, electrolyte imbalances, superinfection, and dehydration. In severe cases initial supportive care may include rehydration, addressing electrolyte abnormalities, and packed red blood cell transfusions. Diagnostic tests to assess the presence and/or severity of the disease include laboratory markers, stool testing, and radiologic imaging. Communication with gastroenterologists and, when needed, surgeons helps to ensure efficient and directed care.
CLINICAL PEARLS AND PITFALLS
• Lower GI bleeding in a child with IBD should not immediately be attributed to a flare of the chronic underlying disease as these children often receive immunosuppressive therapies and are at high risk for infections (e.g., Clostridium difficile).
• In an ill-appearing child with Crohn’s disease and fever, the clinician should consider intra-abdominal abscess and perforation in the differential diagnosis.
• Children with IBD may have extraintestinal manifestations including arthralgia, arthritis, muscle diseases, erythema nodosum, and ocular manifestations such as uveitis.
• Narcotic and anticholinergic usage should be avoided when possible in children with IBD colitis to avoid toxic megacolon.
• Pancreatitis should be considered with acute worsening of abdominal pain in children with IBD, particularly those on thiopurine (e.g., azathioprine or 6-MP) therapy.
Current Evidence. IBD is primarily used to describe two chronic lifelong intestinal disorders: (i) Ulcerative colitis (UC), characterized by inflammation and ulceration confined to the colonic mucosa; and (ii) Crohn’s disease, manifested by transmural inflammation that may affect any segment of the GI tract. The incidence of IBD, particularly of Crohn’s disease, has been increasing in children and adults with an estimated 1.17 million Americans having IBD. The etiology of IBD is unclear but is thought to result from a confluence of host genetic makeup and environmental factors. One of the most important risk factors is a family history of the disease.
IBD likely results from the inappropriate and ongoing activation of the GI mucosal immune system driven by the presence of normal bacterial flora. Growth failure is common in children with IBD. The cause of growth failure in patients with IBD is multifactorial, but inadequate nutrient intake is most likely the final common pathway. Malabsorption, especially with small bowel involvement of the disease, may also occur and micronutrient deficiencies may result in part from the area of intestine affected (e.g., duodenal inflammation is associated with iron deficiency). Hematochezia, protein-losing enteropathy, and increased fecal losses of cellular constituents result from chronic inflammation and damage to the intestinal mucosa. The cause of diarrhea is also multifactorial, resulting from extensive mucosal dysfunction, bile acid malabsorption in terminal ileal disease, bacterial overgrowth secondary to strictures and disordered motility, and protein exudation from inflamed surfaces. Extraintestinal manifestations of the disease are often partially the result of a breakdown in the normal barrier and immunoregulatory functions of the GI tract as a result of chronic inflammation.
Clinical Considerations
Clinical Recognition. Clinical manifestations of IBD can be varied and related to either GI inflammation or the development of either GI tract or extraintestinal complications. Many clinical features are common to both UC and Crohn’s disease, including diarrhea, GI blood and protein loss, abdominal pain, fever, anemia, weight loss, and growth failure. Children with UC are more likely to present with rectal bleeding, diarrhea, and tenesmus. In contrast, children with Crohn’s disease are more likely to present with chronic abdominal pain, weight loss, and failure to thrive. Perianal disease, including fissures, skin tags, fistulae, and abscesses, occurs in 15% of children with Crohn’s disease but not in UC. Perianal disease may precede the appearance of the intestinal manifestations of Crohn’s disease by several years. Extraintestinal manifestations involving the joints (arthritis), skin (erythema nodosum), eyes (uveitis), and liver (chronic hepatitis or sclerosing cholangitis) are seen with both disorders, although they are generally more common with Crohn’s disease.
Abdominal pain and diarrhea with or without occult blood are the most common symptoms at presentation. The pain is often colicky and occurs soon before or during bowel movements in UC. In Crohn’s disease, given possible ileal involvement, the abdominal pain may localize to the right lower quadrant. The abdominal examination may elicit guarding and rebound tenderness prompting consideration of acute appendicitis in the differential diagnosis. Eliciting a history of chronic symptoms and identification of inflammation of other portions of intestine beyond the appendix alone during the evaluation may aid in differentiating IBD from appendicitis.
IBD occasionally causes massive upper or lower GI bleeding due to intestinal mucosal breakdown and potential involvement of blood vessels underneath. Rarely, due to the ability of Crohn’s disease to cause fibrostenosing inflammation, children may present with complete intestinal obstruction. Partial obstruction from significant intraluminal narrowing from stricturing disease, often in the ileum, is more common. Children with Crohn’s disease may develop intra-abdominal abscesses or fistulas due to the transmural nature of the inflammation.
The presence of significant abdominal distention, accompanied by diminished or absent bowel sounds, should raise the suspicion of actual or impending perforation, even in the absence of severe pain. Perforation may occur even after minor abdominal trauma and must be ruled out when patients with known IBD complain of abdominal pain after trauma.
In addition the development of massive colonic distention, termed toxic megacolon, is a rare complication of both UC and Crohn’s disease. Toxic megacolon represents a life-threatening emergency that has a reported mortality rate of as high as 25%. Although rare in children, up to half of the cases occur with the first attack of IBD; another 40% are seen in patients receiving high-dose steroid therapy for fulminant colitis. Toxic megacolon almost always involves the transverse colon. The pathophysiology is believed to be an extension of the inflammatory process through all layers of the bowel wall, with resulting microperforation, localized ileus, and loss of colonic tone. The result is imminent major perforation, peritonitis, and overwhelming sepsis. Antecedent barium enema, opiates, or anticholinergics may all precipitate toxic megacolon. Clinical features include (i) a rapidly worsening clinical course usually associated with fever, malaise, and even lethargy; (ii) abdominal distention and tenderness usually developing over a few hours or days; (iii) a temperature of 38.5°C (101.3°F) or higher and a neutrophilic leukocytosis; and (iv) an abdominal radiograph showing distention of the transverse colon of more than 5 to 7 cm. In a recent case-control study of children with toxic megacolon, fever, tachycardia, dehydration, and electrolyte abnormalities were significantly more common than in age-matched controls with UC without toxic megacolon. The differential diagnosis of acute fulminant colitis includes acute bacterial enteritis, amebic dysentery, ischemic bowel disease, and radiation colitis.
Other potential clinical manifestations of IBD related to extraintestinal complications include thrombosis of cerebral, retinal, or peripheral vessels that may lead to coma, seizures, or focal visual or motor deficits; gallstone cholecystitis; renal calculi leading to hematuria; and pancreatitis. Pancreatitis, in particular, should be considered in an IBD patient on thiopurine maintenance therapy.
Triage. Children with initial presentations of significant GI hemorrhage, toxicity from toxic megacolon, severe dehydration or perforation will be triaged rapidly with standard triage protocols. Children with known IBD presenting with symptoms of a flare (e.g., abdominal pain and diarrhea) with a fever should be evaluated more promptly. The differential includes infectious etiology (e.g., C. difficile), toxic megacolon, or intra-abdominal abscess (more so in Crohn’s disease). C. difficile in particular has become a significant clinical challenge in children with IBD with increased amounts of colonization and infection being reported. Children with signs of orthostasis, dehydration, significant abdominal pain, or active lower GI bleeding may also need to be evaluated promptly.
Initial Assessment/H&P. The initial medical history should be detailed, including family history, for any potential new diagnoses of IBD. Physical examination should include ophthalmic, skin, joint, and perianal evaluation in addition to a comprehensive abdominal examination. In those with an established diagnosis of IBD, medication compliance and identification of medications used for the disease should be completed, particularly those which are immunosuppressive (e.g., steroids). The H&P should also be directed to identify other potential etiologies associated with painful rectal bleeding including intussusception, Henoch–Schönlein purpura (HSP), and hemolytic uremic syndrome (HUS).
Management/Diagnostic Testing. Abdominal radiography, including an upright view, is indicated in cases of suspected partial or full obstruction, toxic megacolon, or perforation. Abdominal ultrasound is the preferred initial modality to evaluate for an abscess, and has a sensitivity approaching 90% and specificity approaching 100% to identify bowel inflammation. Computed tomographic (CT) imaging should be used sparingly and only if urgent information related to inflammation or a possible extraenteric complication is needed, as children with IBD have been identified as having moderately increased exposure to radiation over the course of their chronic disease. MRI enterography has gained favor for a nonradiation method when further delineation beyond ultrasound is desired and may be completed once the child is admitted or in the outpatient setting.
LABORATORY TESTING. Laboratory evaluation should include a CBC with differential, chemistry panel (chem 10, especially in those with chronic diarrhea or vomiting), liver panel (albumin, protein, aminotransferases, bilirubin), erythrocyte sedimentation rate (ESR), C-reactive protein, amylase, and lipase. ESR is elevated in up to 80% of patients with newly diagnosed Crohn’s disease and in 60% of those with newly diagnosed UC. It may also be used to assess the efficacy of therapies in those with previously diagnosed IBD. A blood type and crossmatch is indicated in cases of suspected or confirmed severe anemia. Stool testing for C. difficile, stool culture, as well as ova and parasites should be obtained. Increasingly, fecal calprotectin, a protein produced by neutrophils, is being used to help diagnose IBD and monitor the severity of inflammation in those with established disease.
MANAGEMENT. Management is guided by the history, physical, and diagnostic testing. The role of the ED physician is to provide supportive care while ensuring a significant medical complication (e.g., significant dehydration, electrolyte imbalance, severe anemia, superinfection) or potential complication requiring surgical intervention (e.g., toxic megacolon, intra-abdominal or perirectal abscess, perforation) is identified and addressed if present.
Initial supportive medical care includes rehydration with crystalloid per established protocols. Blood transfusions may be required in those with severe anemia. If toxic megacolon or perforation is suspected, arrangements should be made for admission to an intensive care unit (ICU) and for surgical consultation. An NG tube should be placed. Patients should be started on aggressive doses of broad-spectrum antibiotics such as piperacillin/tazobactam. Suitable alternative therapies may include ampicillin/sulbactam, or cefoxitin in combination with gentamicin.
Management of significant GI bleeding should be performed as described in Chapter 28 Gastrointestinal Bleeding. Emergency management of suspected intestinal obstruction includes gastric decompression with NG drainage and IV rehydration, initially with normal saline. Prompt surgical consultation is required in cases of perforation or toxic megacolon. Concomitant consultation by a pediatric gastroenterologist and surgeon may be indicated with identification of an intra-abdominal or perirectal abscess, fistulizing disease, and partial or complete obstruction to coordinate care during an admission.
Clinical Indications for Discharge or Admission
 The diagnosis of IBD is based on a combination of clinical, pathologic, and radiologic data. In those cases where a medical or surgical emergency is not identified, and diagnostic testing has been completed or started (e.g., stool infectious workup), then discharge of a child with suspected IBD with close follow-up by a pediatric gastroenterologist is appropriate. Further diagnostic studies such as esophagogastroduodenoscopy, colonoscopy, or GI contrast studies can be arranged on an outpatient basis. The initiation of therapies such as corticosteroids, aminosalicylic acid compounds (e.g., mesalamine), immunomodulators (e.g., 6-mercaptopurine), or biologics (e.g., infliximab) can started on an outpatient basis, often after confirmation of the diagnosis.
The diagnosis of IBD is based on a combination of clinical, pathologic, and radiologic data. In those cases where a medical or surgical emergency is not identified, and diagnostic testing has been completed or started (e.g., stool infectious workup), then discharge of a child with suspected IBD with close follow-up by a pediatric gastroenterologist is appropriate. Further diagnostic studies such as esophagogastroduodenoscopy, colonoscopy, or GI contrast studies can be arranged on an outpatient basis. The initiation of therapies such as corticosteroids, aminosalicylic acid compounds (e.g., mesalamine), immunomodulators (e.g., 6-mercaptopurine), or biologics (e.g., infliximab) can started on an outpatient basis, often after confirmation of the diagnosis.
 Psychosocial factors such as concern for possible negligence by the child’s guardians, poor history of follow-up, or significant abdominal pain which cannot be managed at home, may also result in hospital admission. Patients with known IBD who are deemed to have mild to moderate flares may only require adjustments of their IBD maintenance regimen and can be discharged from the ED with close follow-up with their gastroenterologist.
Psychosocial factors such as concern for possible negligence by the child’s guardians, poor history of follow-up, or significant abdominal pain which cannot be managed at home, may also result in hospital admission. Patients with known IBD who are deemed to have mild to moderate flares may only require adjustments of their IBD maintenance regimen and can be discharged from the ED with close follow-up with their gastroenterologist.
 Severe anemia requiring red blood cell transfusion, failure to maintain adequate hydration, electrolyte imbalances requiring rehydration and replacement, and severe failure to thrive are indications for supportive care and admission. Those children with suspected IBD or known IBD in whom a significant medical or surgical manifestation has occurred should be admitted to the appropriate care setting. Children with suspected severe colitis and frequent bloody bowel movements but with normal hemoglobin values should be considered for admission if close follow-up cannot be arranged.
Severe anemia requiring red blood cell transfusion, failure to maintain adequate hydration, electrolyte imbalances requiring rehydration and replacement, and severe failure to thrive are indications for supportive care and admission. Those children with suspected IBD or known IBD in whom a significant medical or surgical manifestation has occurred should be admitted to the appropriate care setting. Children with suspected severe colitis and frequent bloody bowel movements but with normal hemoglobin values should be considered for admission if close follow-up cannot be arranged.
 Children with known IBD with a significant flare may be admitted for modification of existing therapies or initiation of new therapies in consultation with the child’s gastroenterologist. There is increasing use of biologics (e.g., infliximab) in children with IBD early in their course in conjunction with immunomodulators (e.g., azathioprine, mercaptopurine) which will translate to ED physicians seeing more children with established IBD on strong immunosuppressive therapies.
Children with known IBD with a significant flare may be admitted for modification of existing therapies or initiation of new therapies in consultation with the child’s gastroenterologist. There is increasing use of biologics (e.g., infliximab) in children with IBD early in their course in conjunction with immunomodulators (e.g., azathioprine, mercaptopurine) which will translate to ED physicians seeing more children with established IBD on strong immunosuppressive therapies.
Dietary Protein Gastrointestinal Disorders
Goals of Treatment
The initial goals of therapy for dietary protein GI disorders are to identify the suspected protein and remove it from the diet. In the vast majority of cases reassurance, making a dietary change recommendation, and ensuring close follow-up is all that is needed.
CLINICAL PEARLS AND PITFALLS
• Lower GI bleeding in an otherwise healthy-appearing infant is most often dietary protein–induced proctocolitis. Complete resolution of bleeding after an effective dietary change may take several weeks.
• Infants with dietary protein disorders may present with vomiting, diarrhea, and/or shock. Food protein–induced enterocolitis syndrome (FPIES) should be of particular consideration when a child presents with one, or especially, multiple episodes of shock.
• Esophageal food impaction may be the first presentation of a child with eosinophilic esophagitis (EE). The diagnosis of EE should be considered in a child with multiple food impactions without a known anatomic contributor.
• Laboratory and stool testing available to the ED physician is not sensitive in these dietary protein disorders but may include peripheral eosinophilia on a CBC with differential. Stool specimens may also demonstrate elevated eosinophils when testing for fecal leukocytes.
Current Evidence
Food allergy affects nearly 8% of children. Dietary proteins may induce significant bowel injury via both IgE and non–IgE-based immunologic mechanisms. Children may present with GER, dysphagia, colic, abdominal pain, and/or constipation. The symptoms correlate with the portion of GI tract affected (e.g., dysphagia is seen in EE). These allergic diseases begin at different ages with FPIES and allergic proctitis affecting primarily infants while EE more typically is seen in early childhood and later. The symptoms resolve after the offending protein(s) are eliminated from the diet.
Clinical Considerations
Clinical Recognition. The typical presentation of milk-protein sensitivity (allergic) colitis is that of acute onset of blood-streaked, mucoid stool in an otherwise well-appearing infant often younger than 3 months. Blood loss is typically limited, so infants do not appear acutely ill or dehydrated. They are afebrile, and weight gain has typically been normal since birth. In contrast, children with FPIES may present with significant vomiting, diarrhea, and dehydration resulting in shock. Children with EE often have other chronic symptoms which mimic GER but are more likely to present to the ED acutely in the case of food impaction.
Initial Assessment/H&P. Dietary history including any recent changes in the child’s diet or, in the case of breast-feeding infants the mother’s diet, should be noted. A past medical history of asthma, eczema, or rhinitis may be helpful in older children. A strong family history of atopy or food allergy may be found. With infants with rectal bleeding, external anal fissures can be ruled out by careful physical examination. Identification of eczema may support the diagnosis.
Management/Diagnostic Testing. Infants with allergic proctocolitis are rarely hemodynamically unstable or seriously ill; therefore, initial ED management is focused on making a presumptive diagnosis based on history and physical examination, initiating appropriate dietary therapy (e.g., partially hydrolyzed formula), and arranging adequate follow-up with the patient’s primary care physician or a pediatric gastroenterologist. One might consider obtaining CBC count with white blood cell (WBC) differential to assess the hemoglobin level and check for eosinophilia in cases which are severe, refractory, or where there is concern for anemia. Examination of stool for blood, fecal leukocytes, bacterial culture should be performed on infants who have proven refractory to dietary therapy or if there is a known exposure. C. difficile may also be considered though infants may be colonized with this organism. Infants who have milk-protein sensitivity colitis will characteristically have leukocytes seen on fecal smear and eosinophils may also be present. In cases of suspected food impaction, a two-view CXR is often obtained but often does not identify the nonradiopaque food item.
Treatment consists of identifying and eliminating the offending protein from the diet. There is generally improvement in symptoms within 72 hours of the dietary change, though complete resolution may take weeks. Guaiac-positive stools may also persist for several weeks.
Mothers of breast-feeding infants may be asked to eliminate milk protein or other suspected culprit proteins from their diet, but breast-feeding can often be continued. Infants receiving cow’s milk-based or soy protein formulas should be changed to a formula containing casein hydrolysate as the protein source. Nutramigen, Pregestimil, and Alimentum are currently available in the United States. Occasionally, in patients with severe allergic colitis or FPIES, an amino acid–based elemental formula, such as Neocate or Elecare, is recommended.
Children with EE presenting with food impaction require removal of the impaction by either gastroenterology, surgery, or ENT, depending on institutional protocol.
Clinical Indications for Discharge or Admission.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







