(lower should remain OFF until after reperfusion & stabilization)
• Foley catheter: Goal urine output >0.5 mL/kg/hr
• PRBC in the OR; may also need FFP
• Management before clamping
• Induction of GA: Try to maintain BPs near baseline (HTN can rupture aneurysm, hypotension can cause myocardial ischemia)
• Control HR (usually with esmolol)
• Double-lumen tube (DLT) for thoracic aneurysm (L-DLT may risk hemorrhage if aneurysm is eroding bronchial wall)
• Consider deepening anesthesia prior to x-clamp to avoid HTN response BP control: Nitroprusside (SNP) causes arteriolar dilation & MAP reduction; nitroglycerin (NTG) may prevent myocardial ischemia & ↓ preload
• Maintain relative hypovolemia during preclamp phase to prevent HTN from inc afterload during x-clamp & ↓ risk of MI during x-clamp (do not overhydrate, use NTG/SNP)
• Preparation for clamp release
• Gradually load with volume
• Wean vasodilators & have pressors ready
• Lighten anesthetic
• Postclamp management
• Give fluid bolus, blood (if warranted)
• Gradual release of clamp can ↓ hemodynamic changes
• If severe hypotension results, reclamp & reassess
• Pressors (phenylephrine) may be needed, although not usually given prophylactically
• ↑ ventilation
• ABG before & after x-clamp removal (guide fluid & electrolyte management)
• Monitor HCT & correct coagulopathies
• Use standard extubation criteria (pts often stay intubated 2° large volume shifts)
• Preventing renal failure
• Risk with supraceliac > suprarenal > infrarenal
• Maintain renal perfusion pressure with highest possible MAP that myocardium will tolerate
• Maintain intravascular volume
• Consider mannitol (0.5 g/kg before x-clamping), furosemide, Ca2+ blockers, dopamine, fenoldopam (not proven effective); bicarb drip
• Preventing spinal cord ischemia
• SSEP monitoring—not useful (2/3 of cord is supplied by anterior spinal artery → motor)
• Maintain highest MAP (distal aortic perfusion pressures) that myocardium can handle
• Keep CSF pressures low (consider spinal fluid drain)
• Consider shunt to maintain distal perfusion during x-clamp
• Consider hypothermic CPB or circulatory arrest
• Consider administering steroids, barbiturates
• Consider epidural cooling
• Spinal cord perfusion pressure (SCPP)
SCPP = distal aortic pressure – (greater of spinal CSF pressure or CVP)
• If monitoring distal pressures, aim for SCPP >30 mm Hg; can drain CSF via lumbar drain, up to ∼15 mL/15 min (risk of brainstem herniation with rapid or excessive CSF drainage → limit to ∼75 mL)
• Avoid excessive SNP (hypotension → ↓ perfusion, cerebral vasodilation → ↑ ICP transmitted to CSF)
• Avoid hyperglycemia (consider insulin infusion for glucose >200)
• Consider mild hypothermia (passive cooling to about 34°C)
• Other complications
• Nerve injuries: Recurrent laryngeal nerve during thoracoabdominal repairs, brachial plexus injuries (poor pt positioning)
Thoracoabdominal Aortic Aneurysm (TAAA) Repair
• Management similar to AAA (see above) with following key points
Crawford Classification of TAAA (I–IV)
• I: Descending thoracic aortic aneurysm distal to subclavian artery
• II: Aneurysm originating at subclavian artery to distal abdominal aorta
• III: Aneurysm from mid–descending thoracic aorta to distal abdominal aorta
• IV: Abdominal aortic aneurysm (below the diaphragm)
Stanford Classification of TAAA (A–B)
• Type A: Intimal tear (acute) in aorta from ascending aorta to descending aorta
• Type B: Intimal tear (acute or chronic) in aorta from descending aorta down
Possible Associated Findings with TAAA
• Airway deviation/compression
• Tracheal deviation/compression
• Hemoptysis
• Esophageal deviation/compression
• Distortion & compression of central vasculature/anatomy
• Hemothorax & mediastinal shift
• Reduced distal perfusion
(Adapted from: Dunn P. Clinical Procedures of the MGH. Philadelphia, PA: Lippincott Williams & Wilkins.)
Anesthetic Management of TAAA
• A-line: Ascending aneurysm, usu. placed in L radial (innominate artery may be involved); descending aneurysm, usu. placed in R radial (left subclavian may be clamped)
• Circ arrest: If utilized, will need to pack head in ice (cover monitors so they remain dry)
• TEE: Used intraop to detect intimal tear, coronary ostia, AI, assess embolic risk
• Neuroprotection: Thiopental 3–10 mg/kg (may offer benefit for cerebral protection)
• Partial bypass: May be used for descending aneurysms
• Ventilation: One-lung ventilation often employed
• Access: 1 large-bore peripheral IV (16- or 14-gauge) + 1 large-bore central line
BP Control During TAAA
• If no bypass: Maintain SBP at baseline SBP + 1⁄2 of peak aortic x-clamp SBP
• If bypass: Maintain SBP at baseline SBP
• Can reduce proximal HTN during aortic clamp by ↑ flow to pump & ↓ flow to heart
• SNP should be used sparingly (or not at all) during aortic clamp (risk of ↓ spinal cord & renal perfusion)
• ↓ conc of volatile agent & turn off vasodilators before aortic unclamp
• Volume repletion with colloid, crystalloid, blood products before & after aortic unclamp
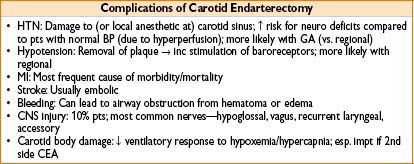
Carotid Endarterectomy
• Indication: History of stroke, TIA, or significant arterial occlusion on angiography
• Morbidity: Incidence of concomitant CAD ≈ 50%; periop mortality 1–4%
• Anesthetic techniques
• Regional advantages
• Pt can tell you of neurologic symptoms/deficits during surgery
• Less anesthesia required for pts with significant comorbidities
• Avoidance of coughing/bucking at case end
• Less postop hyper- & hypotension
• Potentially reduced ICU & hospital stay
• Regional disadvantages
“A good general is always better than a bad regional” (if regional not working, pt may be uncomfortable, moving, & tachycardic)
Some providers give “deep sedation” + regional anesthesia
(eliminates benefit of awake detection of neurologic deficits)
• Regional: Deep cervical block
• Technique: Inject anesthetic at C2, C3, C4 in line drawn between mastoid process, & C6 transverse process; needle should have slight caudal angulation, contact transverse process, withdraw 2 mm & inject
• Potential complications:
Intravertebral artery injection
Horner’s syndrome (sympathetic chain)
Hoarseness (recurrent laryngeal nerve)
• Regional: Superficial cervical block
• Technique: Inject anesthetic just posterior to sternocleidomastoid (goal to spread anesthetic subcutaneously & behind SCM) at C6 level, & fanned 2–3 cm superior & inferior
• Easy technique with minimal risk & excellent efficacy
• General anesthesia: Advantages
• Potential for brain protection by volatile and intravenous anesthetics
• General anesthesia: Disadvantages
• Necessitates careful planning & drug management to avoid HTN, coughing, & bucking during emergence & extubation
• Can get hypotension (minimal surgical stim but must keep pt still)
• No proven mortality ↓ with either technique (GA vs. regional)
• Intraoperative shunting
• Provides blood flow from common carotid artery to internal carotid artery (distal/superior to site of x-clamp)
• Indicated in pts with significant contralateral dz
• Stump pressure: Measurement of pressure distal to site of x-clamp, need to provide well-flushed A-line tubing over drape stump pressure <50 mm Hg = indication for shunting
• Risk of plaque dislodgement, intimal injury, & air embolus
• Hemodynamic management
• Avoid tachycardia (↑ myocardial O2 demand) & hypotension (↓ coronary flow)
• Maintain MAP slightly above baseline (optimizes collateral blood flow)
May be difficult to maintain normal MAP (minimal surgical stim)
Phenylephrine infusion → ideal to maintain MAP without raising heart rate
• Consider nitroglycerin for reduction of BP at induction/emergence
Esp in chronically HTN pts (may have wide swings in MAP)
• Consider esmolol/metoprolol to prevent tachycardia
Intubation, reversal of neuromuscular blockade, extubation
• Consider A-line placement prior to induction in pts with known CAD
• Intraoperative brain monitoring has not been shown to improve outcomes
• CNS monitors:
• Awake: ↓ cardiac morbidity & HTN, shorter ICU stay
• EEG: May correlate with neuro changes
• SSEPs: Sensitive, but intermittent indicator of cortical ischemia
• Stump press poor sensitivity/specificity
• Transcranial Doppler/brain oximetry/JvO2 (unproven)
• Perioperative complications
• Brain hypoperfusion (avoid hyperglycemia)
• Bradycardia (esp during carotid body manipulation)
Can avoid with lidocaine infiltration by surgeon
• Intraoperative stroke (consider if delayed emergence/mental status change)
• Hematoma: Evacuate hematoma 1st, manipulate airway 2nd
• Diagnosis: Progressive stridor & subjective difficulty breathing; often difficult to see hematoma (dressings/patient size)
• Treatment: Pt back to OR stat—if condition worsening, open wound prior to airway manipulation; attempts at intubation can be impossible (may result in airway swelling/bleeding, making situation worse)
ENDOVASCULAR PROCEDURES
Endovascular AAA Repair
• Monitoring for most limited to A-line (plus large-bore IV access)
• Pressors/vasodilators usually not needed
• Conversion to open procedure rate <5% (should always anticipate this possibility)
• Anesthetic options
• General
• Complex cases (inexperienced surgeon) or pt refuses regional/MAC
• Always considered as backup for conversion to open procedure
• Regional
• Spinal: Duration of procedure usually precludes this
• Epidural: Allows for ideal anesthesia of incision sites (bilateral femoral vascular access), But must be prepared to delay case if achieve bloody/traumatic tap or intravascular catheter
• Regional techniques may ↓ incidence of hypercoagulability & perioperative vessel clot formation (esp for lower extremity procedures)
• Sedation
• Ideal for thin pts (less dissection necessary) if surgeons apply local
• Pt must remain still for hrs on uncomfortable fluoroscopy bed
• Contrast induced nephropathy a concern (2° to extensive angiography) (see below)
Carotid Stent Placement
• Requires immobile pt (minimal head/neck movement) & able to tolerate fluoroscopy table
• Consider narcotic/α-2 agonist technique (may avoid sedation-associated confusion)
Distal Angioplasty/Thrombectomy
• Pts with operative lower limb vascular dz have >50% incidence of concomitant CAD
• Procedure times often long (on uncomfortable fluoroscopy bed) usually best to avoid long infusions/large doses of midazolam/propofol (problem of confusion/disorientation)
• Always be prepared for conversion to open procedure
• Regional techniques may ↓ incidence of hypercoagulability & perioperative vessel clot formation (esp for lower extremity procedures)
ENDOVASCULAR SAFETY CONCERNS
• Perioperative β-blockade: Current ACC guidelines – recommend perioperative β-blockade in vascular pts found to have myocardial ischemia on preop testing
(less strong evidence for pts with low/intermediate cardiac risk)
• Transfusion triggers: Evidence suggests vascular pts allowed to bleed below a hemoglobin level of 10 mg/dL have ↑ incidence of periop myocardial ischemia
• Regional anesthesia & anticoagulation (see Chapter 6, Regional Anesthesia)
CONTRAST-INDUCED NEPHROPATHY (CIN)
• ARF after ischemia or contrast thought 2° to acute tubular necrosis from
• Free-radical formation, which is promoted in acidic environment (e.g., renal medulla)
• Contrast-related ↓ in renal blood flow
• Atheroembolism
• Tips to Avoid CIN
• Maintain plasma volume, good urine output
• NaHCO3 may be protective: D5 NaHCO3 154 mEq/L (from pharmacy)
• Load: 3 mL/kg over 1 hr, given 1 hr before contrast
• Maintenance: 1 mL/kg/hr until 6 hr after procedure
• Use 110 kg max weight for calculations
• If bolus leads to significant HTN → stop bolus, diurese before injecting contrast, then resume infusion
• N-acetylcysteine (free-radical scavenger)
• 600 mg PO bid starting day before surgery and through day of surgery
• Risk Factors
• Patient factors: Renal dz, diabetes, CHF, ↑ age, anemia, LV dysfx
• Nonpatient factors: ↑ osmolar or ionic contrast, contrast viscosity & volume
PERIPHERAL VASCULAR SURGERY
• Preop risk: Patients often have significant comorbidities (↑ risk of associated CAD)
• Procedures: Bypass grafts (fem-pop, ilio-fem, etc.), embolectomy, pseudoaneurysm repair
• Monitoring: Invasive monitors per pt condition (hemodynamics often labile)
(place A-line in side opposite surgery)
• Anesthetic
• General anesthesia/regional/MAC
• Epidural & GA → associated with comparable rates of cardiac morbidity
• Continuous epidural/spinal
• ↓incidence of postop vascular graft clotting (Anesthesiology 1993:79:422)
• Continuous lumbar epidural catheter commonly used (occ spinal)
• Awake pts can notify personnel of acute MI symptoms (chest pain)
• Helpful for postop pain control
• Intraop heparin after epidural placement does not ↑ risk of epidural hematoma
• Epidural associated with ↓ incidence of reoperation for inadequate tissue perfusion (compared to GA) (Anesthesiology 1993;79[3]:422–434)
< div class='tao-gold-member'>









