Fig. 12.1
Topography and constrictions of the esophagus (F. Netter. Atlas of Human Anatomy. 4th Edition, Saunders Elsevier, 2006, Philadelphia, PA. page 233)
The absence of a serosal layer in the esophagus increases risk of perforation and, when perforation occurs, adds greater likelihood of bacterial contamination.
12.1.2 Esophageal Microflora and Appropriate Antimicrobials
A mixed aerobic and anaerobic microflora inhabits the esophagus. Streptococci, Staphylococci, Klebsiella pneumoniae, and Escherichia coli predominate. The anaerobic species include Prevotella, Porphyromonas, Bacteroides fragilis, Fusobacterium, and Peptostreptococcus, in addition to frequent colonization with yeast found in obstructive diseases.
The optimal antimicrobial treatment in esophageal perforation is broad-spectrum antimicrobials such as cefoxitin sodium, clindamycin phosphate, beta-lactamase-resistant penicillins, and antifungal agents when appropriate.
12.2 Esophageal Perforation
The most frequent cause of esophageal perforation is instrumentation.
Diagnostic flexible endoscopy carries a relatively low overall perforation risk of 1:3000. Despite the low risk, it is a widely used diagnostic modality resulting in a significant number of esophageal perforations.
Diagnostic interventions such as Maloney bougienage, Savary pneumatic dilatation, through-the-endoscope hydrostatic balloon dilators, Sengstaken-Blakemore tube deployment, sclerotherapy, and banding in esophageal varices, and endotracheal intubation increase the risk of perforation, particularly in patients with esophageal pathology.
Dilatation in achalasia and strictures carry relatively higher perforation rates at 2–6 % and 0.3 %, respectively.
Esophageal perforation can occur also after surgical interventions such as fundoplication, esophageal myotomy, vagotomy, lung resection, thyroid surgery, tracheostomy, chest tube placement, mediastinoscopy, or spine surgery.
Spontaneous rupture of the esophagus caused by voluminous vomiting or retching is named after Dutch physician Hermann Boerhaave who described the condition in 1724.
The classic presentation of spontaneous perforation includes sudden retrosternal pain radiating to the neck associated with tachycardia and tachypnea.
Hematemesis is rarely seen in spontaneous perforation which helps distinguish it from the Mallory-Weiss tear.
Spontaneous rupture has been observed following blunt chest trauma, severe coughing, weightlifting, and childbirth.
12.2.1 Assessment and Diagnosis
The initial investigation includes history, examination, and chest radiography.
Signs and symptoms
Severe pain is the keystone manifestation of esophageal perforation.
Cervical or thoracic perforation is associated with sudden sharp pain in the neck or substernal area, respectively, shortly after the spontaneous perforation or esophageal instrumentation.
In addition, odynophagia, dysphagia, fever, subcutaneous emphysema, hemoptysis, or blood in nasogastric tube may be present.
Chest X-ray
Shows nonspecific findings such as mediastinal emphysema or pleural effusion in majority of cases (90 %).
An unexplained pleural effusion on chest radiography is suspicious for esophageal perforation.
Commonly, the perforation is confirmed with contrast esophagography (Fig. 12.2). The study is performed initially with water-soluble gastrografin because it is less harmful if it leaks into the mediastinum. However, if aspirated into the tracheobronchial tree or leaks through a tracheoesophageal fistula into the lungs, gastrografin may cause severe pneumonitis.
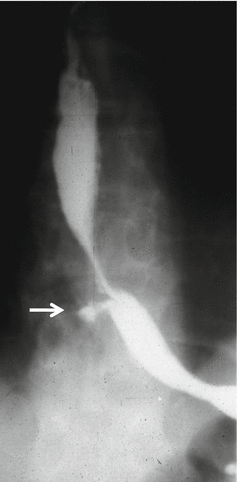
Fig. 12.2
Thoracic esophageal perforation noted on esophagography (arrow)
The optimal diagnostic accuracy is obtained with thin barium esophagography performed in decubitus position for slower-contrast transit time through the esophagus. Barium contrast has superior radiologic density and delineates mucosa better. With negative good-quality barium study, the perforation is unlikely.
Multidetector computed tomography (MDCT) and MDCT esophagography has shown a high accuracy for esophageal perforations. Screening chest MDCT may depict mediastinal air, air-fluid collections communicating with esophageal air, or an abscess adjacent to esophagus. Directed MDCT esophagography may confirm perforation demonstrating leakage of contrast media into mediastinum or pleural space. In patients with empyema, thoracentesis may yield pus-containing food particles.
In summary, a high index of suspicion must be maintained in suspected esophageal perforation because outcome depends entirely on timely diagnosis and prompt management.
12.2.2 Treatment
Therapeutic options in esophageal perforation depend on the anatomical site of the perforation, underlying pathology, time to diagnosis, signs of severe sepsis, and extent of the perforation and leak.
Although surgery is the gold standard in the management of esophageal perforation, not all esophageal perforations require operative management.
12.2.2.1 Nonoperative Management (NOM)
Is feasible in patients suffering small, contained perforations with no signs of sepsis, especially in the cervical esophagus
Criteria for NOM include intramural perforation, contained perforation communicating with esophagus, non-stricture and nonmalignant perforation associated with mild degree of sepsis.
Some recent evidence advocates nonoperative “aggressive conservatism” that includes antibiotic treatment, aggressive drainage of mediastinal and chest collections, and sequential imaging
The principles of NOM include restriction of oral intake, broad-spectrum antibiotics, CT-guided drainage of any fluid collections in the neck or the mediastinum, and parenteral nutrition.
Endoscopically placed stents in selected cases have been used with good success (Fig. 12.3).
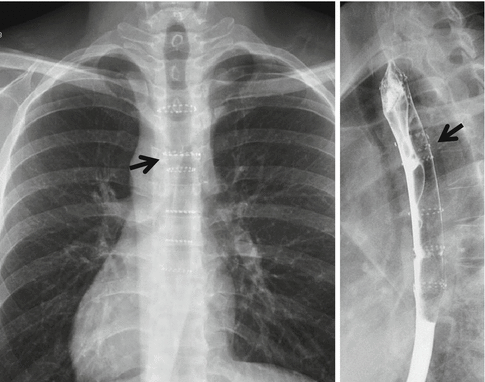
Fig. 12.3
Endoscopically placed stent in the esophagus (arrow)
12.2.2.2 Operative Management
Surgery
The cornerstone of therapy in uncontained perforations and in all patients with severe sepsis or septic shock.
Options include drainage alone, primary repair, diversion, or esophagectomy depending on the site of perforation, pathology, severity of sepsis, and the interval from perforation to diagnosis. While immediate diagnosis of perforation without preexisting pathology allows primary repair, in those patients with preexisting disease or delay in diagnosis, primary repair will likely fail with devastating septic complications. Even after meticulous surgical repair, leak rates range from 25 % to 50 % mandating placement of closed suction drains near the repair.
Surgical Approaches
Cervical Esophagus
The cervical esophagus is relatively easy to approach using the left unilateral sternocleidomastoid incision.
Two-layer repair should be used: a running absorbable suture to the mucosa followed by an interrupted suture line to the muscular layer.
It is crucial to extend the myotomy to assess the entire mucosal length of the defect.
The mucosal primary repair can be carried out over a large bougie.
The sternocleidomastoid or omohyoid muscle can be placed over the repair
There is no evidence that nasogastric tube following cervical esophageal repair provides diversion of saliva and may compromise the tenuous repair and healing of the wound.
Thoracic Esophagus
Thoracic esophageal perforations are repaired through a right fourth to fifth or a left sixth to seventh intercostal posterolateral thoracotomy (Fig. 12.4).
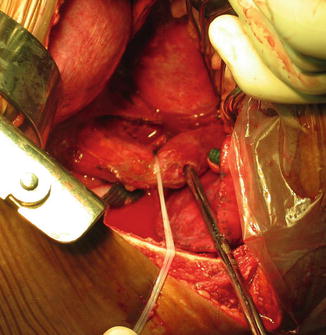
Fig. 12.4
Left posterolateral thoracotomy, suction tube passed through an esophageal perforation
Running absorbable suture line for mucosa and interrupted absorbable for muscular layer is appropriate.
In mid and lower esophageal repairs, a diaphragmatic buttress flap can be utilized.
For that purpose, rotation-flap constructed from the posterior aspect of diaphragm is sutured over the esophageal repair. The diaphragmatic defect is primarily closed.
In preexisting disease without significant contamination, resection of a tumor, myotomy for strictures, or antireflux procedure may be feasible.
Esophagectomy with primary reconstruction.
May be successful in minimal contamination.
In severe contamination and inflammation, diversion is appropriate. Closure of the perforation with proximal and distal staple line, resection of the diseased segment, and a proximal esophagostomy is established with wide drainage and gastrostomy tube for feeding. Major esophageal reconstruction is required at later stage.
Another option includes closure of the wound over a 24-French T-tube drainage brought out to the chest wall and placement of chest drainage (Fig. 12.5).
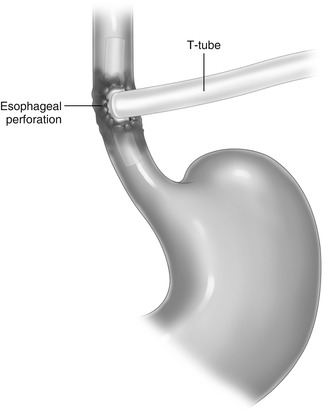
Fig. 12.5
Illustration showing T-tube drainage of an esophageal perforation not amenable for safe repair
Abdominal Esophagus
Abdominal esophagus is accessed via laparotomy or more often via a thoracoabdominal incision.
A self-retaining retractor system is of great value for optimal exposure of the gastroesophageal junction.
The defect with minor contamination is debrided and repaired in two layers added by buttressing with a stomach patch around the repair site. More destructive injuries may require resection of the affected segment, mobilization of the stomach, and esophagogastrostomy in the chest.
In benign conditions with extensive tissue loss, resection of the esophagus and reconstruction with colon interposition may be needed.
12.3 Caustic Ingestion
Most commonly encountered in children and in young adults when ingestion is accidental or intentional, respectively.
The degree of injury depends on the nature of the ingested substance, concentration, quantity, and duration of the caustic agent exposure.
Hence, adults frequently sustain more severe injuries as the ingested volumes are larger.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







