ENDOCRINE EMERGENCIES
MICHAEL S.D. AGUS, MD AND KATE DORNEY, MD
KEY POINTS
DKA/Hyperglycemia
 1% of children with diabetic ketoacidosis will develop cerebral edema
1% of children with diabetic ketoacidosis will develop cerebral edema
 Risk factors for cerebral edema include elevated blood urea nitrogen, low Pco2, treatment with bicarbonate, failure of serum Na to rise steadily with correction of hyperglycemia, age <3 years, new-onset diabetes
Risk factors for cerebral edema include elevated blood urea nitrogen, low Pco2, treatment with bicarbonate, failure of serum Na to rise steadily with correction of hyperglycemia, age <3 years, new-onset diabetes
 Hyperglycemia in ED setting can result from numerous triggers including intercurrent illness or trauma in patient with known DM, new-onset DM, other illnesses associated with hyperglycemia, spurious sample, medication effect
Hyperglycemia in ED setting can result from numerous triggers including intercurrent illness or trauma in patient with known DM, new-onset DM, other illnesses associated with hyperglycemia, spurious sample, medication effect
Hypoglycemia
 Prompt recognition of hypoglycemia is important to avoid adverse outcomes
Prompt recognition of hypoglycemia is important to avoid adverse outcomes
 Hypoglycemia in absence of ketones is consistent with hyperinsulinism or fatty acid oxidation enzyme deficiencies
Hypoglycemia in absence of ketones is consistent with hyperinsulinism or fatty acid oxidation enzyme deficiencies
Hypopituitarism
 The acute presentation of hypopituitarism is most likely to occur when the child is stressed by injury, illness, or fasting
The acute presentation of hypopituitarism is most likely to occur when the child is stressed by injury, illness, or fasting
 Children with hypopituitarism are prone to hypoglycemia
Children with hypopituitarism are prone to hypoglycemia
 Hypopituitarism is associated with intracranial developmental anomalies or lesions
Hypopituitarism is associated with intracranial developmental anomalies or lesions
Adrenal Insufficiency/Congenital Adrenal Hyperplasia
 Cortisol and aldosterone replacement in patients with adrenal insufficiency under stress conditions is imperative
Cortisol and aldosterone replacement in patients with adrenal insufficiency under stress conditions is imperative
 ED presentations include addisonian crisis, ambiguous genitalia, acute salt-wasting crisis, and precocious puberty
ED presentations include addisonian crisis, ambiguous genitalia, acute salt-wasting crisis, and precocious puberty
 Patients with acute salt-wasting crisis must be recognized and treated immediately with fluid resuscitation, stress dose hydrocortisone, careful monitoring of electrolytes
Patients with acute salt-wasting crisis must be recognized and treated immediately with fluid resuscitation, stress dose hydrocortisone, careful monitoring of electrolytes
Pheochromocytoma
 Often presents with headache, palpitations, sweating; but also nervousness, tremulousness, fatigue, chest/abdominal pain, and flushing
Often presents with headache, palpitations, sweating; but also nervousness, tremulousness, fatigue, chest/abdominal pain, and flushing
 Most associated with hypertension although can be paroxysmal; alpha blockade is antihypertensive of choice, avoid pure beta blockade as can lead to severe hypertension
Most associated with hypertension although can be paroxysmal; alpha blockade is antihypertensive of choice, avoid pure beta blockade as can lead to severe hypertension
Diabetes Insipidus
 Inability of kidneys to concentrate urine resulting in polyuria/polydipsia
Inability of kidneys to concentrate urine resulting in polyuria/polydipsia
 Hypertonic dehydration if thirst is not intact or access to fluids is restricted; if occurs abruptly, patient at risk for central pontine myelinolysis
Hypertonic dehydration if thirst is not intact or access to fluids is restricted; if occurs abruptly, patient at risk for central pontine myelinolysis
 Consider in patients with increased urine excretion, enuresis, and increased thirst
Consider in patients with increased urine excretion, enuresis, and increased thirst
 Management is similar to hypernatremic dehydration with initial reexpansion of intravascular volume, then repletion of maintenance over 48 hours
Management is similar to hypernatremic dehydration with initial reexpansion of intravascular volume, then repletion of maintenance over 48 hours
Syndrome of Inappropriate Antidiuretic Hormone Secretion
 Associated with bacterial meningitis (50%), positive pressure ventilation (20%), Rocky Mountain spotted fever (70%), and numerous other illnesses
Associated with bacterial meningitis (50%), positive pressure ventilation (20%), Rocky Mountain spotted fever (70%), and numerous other illnesses
 For severe lethargy, seizure, or coma administer 3% saline emergently (3 mL per kg every 10 to 20 minutes as needed), consider furosemide, initiate antiepileptic drugs if indicated, and treat underlying cause
For severe lethargy, seizure, or coma administer 3% saline emergently (3 mL per kg every 10 to 20 minutes as needed), consider furosemide, initiate antiepileptic drugs if indicated, and treat underlying cause
Hyperparathyroidism
 Uncommon in children
Uncommon in children
 Family history is important as hyperparathyroidism is associated with MEN I, II and being an infant born to a mother with hypoparathyroidism
Family history is important as hyperparathyroidism is associated with MEN I, II and being an infant born to a mother with hypoparathyroidism
 Demineralization and bone resorption seen on x-ray
Demineralization and bone resorption seen on x-ray
Hypoparathyroidism
 Rare in children but tends to be associated with familial autoimmune syndromes, immunologic deficiencies, or iatrogenic etiologies
Rare in children but tends to be associated with familial autoimmune syndromes, immunologic deficiencies, or iatrogenic etiologies
Rickets
 Rickets is caused by inadequate dietary intake of vitamin D; incidence is decreasing as increased awareness and increased supplementation in food
Rickets is caused by inadequate dietary intake of vitamin D; incidence is decreasing as increased awareness and increased supplementation in food
 Failure of calcification affects those parts of the skeleton that are growing most rapidly or that are under stress; a clinical diagnosis that is confirmed by radiology
Failure of calcification affects those parts of the skeleton that are growing most rapidly or that are under stress; a clinical diagnosis that is confirmed by radiology
Thyroid Storm
 Thyroid storm is the fulminant intensification of hyperthyroid state
Thyroid storm is the fulminant intensification of hyperthyroid state
 Precipitated by intercurrent infection, trauma, or after subtotal thyroidectomy with an inadequately prepared patient
Precipitated by intercurrent infection, trauma, or after subtotal thyroidectomy with an inadequately prepared patient
 Presence of high fever (often to 105.8°F [41°C]) is primary distinguishing feature of Thyroid Storm from hyperthyroid
Presence of high fever (often to 105.8°F [41°C]) is primary distinguishing feature of Thyroid Storm from hyperthyroid
 Marked increase in cardiac workload may result in high-output heart failure and hypotension and pulmonary edema rather than classic hypertension
Marked increase in cardiac workload may result in high-output heart failure and hypotension and pulmonary edema rather than classic hypertension
Neonatal Thyrotoxicosis
 Neonatal thyrotoxicosis is a life-threatening condition found in 1% to 5% of infants born to mothers with history of hyperthyroidism; mother’s thyroid disease does not have to be active during pregnancy
Neonatal thyrotoxicosis is a life-threatening condition found in 1% to 5% of infants born to mothers with history of hyperthyroidism; mother’s thyroid disease does not have to be active during pregnancy
Congenital Hypothyroidism
 Increased incidence of ED visits for congenital hypothyroidism now that infants are being routinely screened at birth
Increased incidence of ED visits for congenital hypothyroidism now that infants are being routinely screened at birth
 Untreated disease may result in impairment of neurologic development
Untreated disease may result in impairment of neurologic development
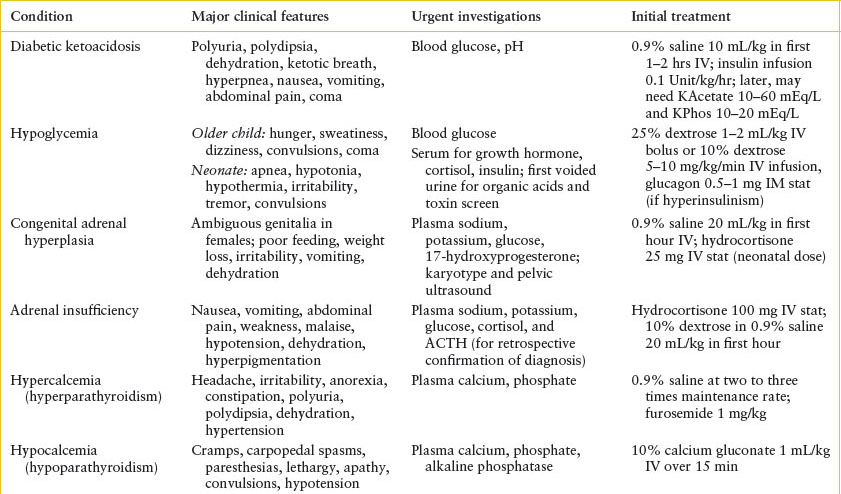
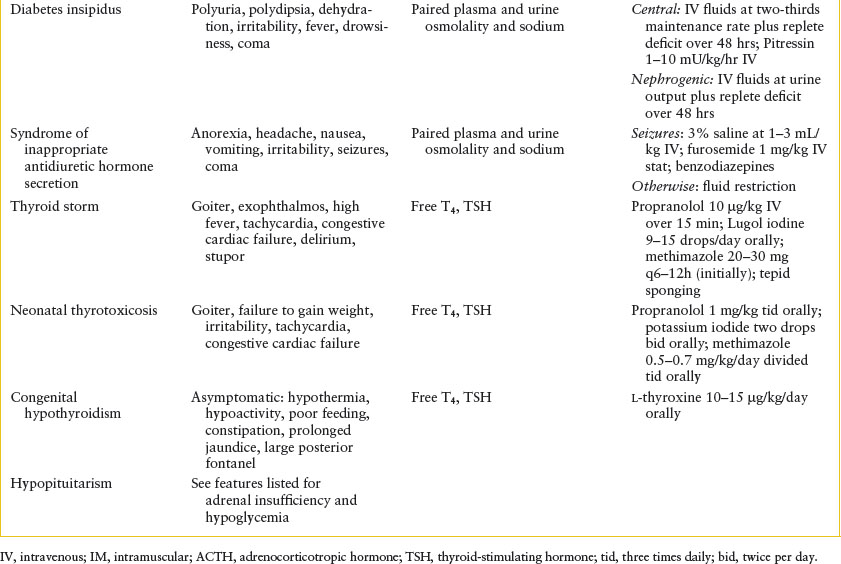
See Table 97.1.
TABLE 97.1
SUMMARY OF CLINICAL FEATURES, INVESTIGATIONS, AND INITIAL TREATMENT OF PEDIATRIC ENDOCRINE EMERGENCIES
DIABETIC KETOACIDOSIS
Goals of Treatment
To identify patients with DKA and initiate treatment per algorithm.
To recognize patients with cerebral edema (1%) and intervene with appropriate treatment.
CLINICAL PEARLS AND PITFALLS
• Clinically significant cerebral edema is the most serious immediate risk to the child, occurring in 1% of cases, and it remains so during the first 24 hours of therapy, despite the more apparent issues of hypovolemia and acidosis.
• The treatment for symptomatic cerebral edema is mannitol and/or 3% saline.
• Avoid bicarbonate administration.
Current Evidence
Insulin deficiency initially leads to hyperglycemia that, once above the renal threshold of 180 mg per dL, leads to polyuria due to an osmotic diuresis. Without vigorous oral repletion at home, the child quickly becomes hypovolemic, prompting a stress response and elevations of the counterregulatory hormones glucagon, cortisol, growth hormone, and catecholamines. These hormonal changes produce significant insulin resistance and stimulate glycogenolysis and gluconeogenesis that worsens the hyperglycemia, hypovolemia, and stress response. In this insulin-deficient state, adipose tissue is broken down in large quantities into free fatty acids, subsequently converted into ketoacids in the liver. Ketoacids readily dissociate in the blood to produce free hydrogen ions, and metabolic acidosis ensues. This reaction is partially compensated for by a respiratory alkalosis (hyperventilation), with a resultant lowering of Pco2 and plasma bicarbonate (HCO3−).
Intracellular potassium is depleted because of transcellular shifts of this ion brought about by the exchange of potassium with excess free hydrogen ions and extracellular dehydration. Protein catabolism secondary to insulin deficiency causes a negative nitrogen balance and results in additional efflux of potassium from cells. The potassium is then lost in the urine during the osmotic diuresis. Volume depletion causes secondary hyperaldosteronism, which further promotes urinary potassium excretion. Thus, total body depletion of potassium occurs, although the plasma potassium concentration may not reflect the loss at the time of presentation.
Clinical Considerations
Clinical Recognition
In cases of new-onset diabetes, the child usually has a history of polyuria and polydipsia for a few days or weeks before the acute decompensation. Significant weight loss often occurs despite a vigorous appetite. Vomiting is common once the child has ketoacidosis; these further losses plus the inability to compensate for polyuria contribute to the hypovolemia.
In children known to have diabetes, the prodrome may be less than 24 hours and precipitated by an intercurrent illness, inappropriate sick day management, or omission of insulin doses.
Triage
On physical examination, particular attention should be paid to the degree of dehydration, including skin turgor and dryness of mucous membranes. Urine output is not a reliable sign of hydration status. In severe cases, the child may exhibit signs of compensated shock, including a thready pulse and cold extremities, and rarely, as uncompensated shock with hypotension. The smell of ketones on the breath and the presence of hyperpneic (Kussmaul) respirations reflect the ketoacidosis. The patient’s consciousness level, which may range from full alertness to deep coma, should be noted.
Initial Assessment/H&P
Patients may complain of nausea, vomiting, and abdominal pain, and the parents may have noticed increasing listlessness. Less than 2% of children are in coma at the time of hospital admission, although a higher percentage has an altered state of consciousness. The history and physical examination usually suggest the diagnosis; however, particularly in the patient with new-onset diabetes, presenting clinical features can be misdiagnosed, especially in the infant or young child. For example, abdominal pain may be misinterpreted as appendicitis; hyperpnea may be mistaken as a sign of pneumonia or asthma; and polyuria may be incorrectly diagnosed as a urinary tract infection. Enuresis, polydipsia, and irritability are sometimes wrongly categorized as behavioral problems. The child may have exquisite abdominal tenderness with guarding and rigidity, which can mimic an acute abdomen. The ears, throat, chest, and urine should be examined because infection is often a precipitating factor. Careful attention should be paid to the skin examination because there have been several case reports of fasciitis copresenting with DKA. The presence of hyperpigmentation (acanthosis nigricans) on the posterior neck is a sign of long-standing insulin resistance and should alert the clinician to the possibility of non–insulin-dependent diabetes.
Management/Diagnostic Testing
Diagnostic laboratory findings include plasma glucose greater than 200 mg per dL (commonly 400 to 800 mg per dL), the presence of glucose and ketones in the urine, and acidosis (venous pH less than 7.3 and serum bicarbonate less than 15 mEq per L). Additionally, high or normal plasma potassium, and slightly elevated blood urea nitrogen are common. Occasionally, DKA can occur with normoglycemia when persistent vomiting and decreased intake of carbohydrates are accompanied by continued administration of insulin or when patients have kept themselves particularly well hydrated with non–glucose-containing fluids. The measured serum sodium is usually low or in the low to normal range. In the setting of hyperglycemia, the measured sodium will be lowered; a commonly used estimate for correction is a decrease of 2 mEq/L Na for every 100 mg per dL elevation in glucose above normal. Leukocytosis with a left shift may be noted but does not necessarily signify an underlying infection. Hyperglycemia in the absence of acidosis should cause the clinician to consider additional possibilities (see Hyperglycemia section).
For the severely dehydrated child, initial treatment is directed toward expansion of intravascular volume and administration of insulin. Subsequent treatment is directed at the normalization of the remaining abnormal biochemical parameters. Medical intervention carries significant risks of hypokalemia and cerebral edema (Tables 97.2 and 97.3).
TABLE 97.2
PRINCIPLES OF MANAGEMENT OF DIABETIC KETOACIDOSIS
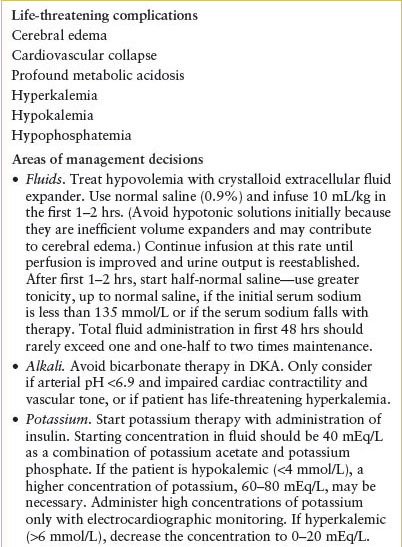

TABLE 97.3
RISK FACTORS FOR CEREBRAL EDEMA IN DIABETIC KETOACIDOSIS

Fluid and Electrolyte Replacement
Fluid replacement should be instituted promptly. In the first 1 to 2 hours, if hypovolemia is apparent, 10 mL per kg isotonic (0.9%) crystalloid (either normal saline or lactated Ringer’s) should be infused intravenously to establish an adequate intravascular volume and improve tissue perfusion. Normal saline is generally preferred for initial resuscitation given that DKA patients already have a degree of lactic acidosis, however, lactated Ringer’s has the benefit of a reduced chloride load. A small head-to-head trial showed no significant differences between the two fluids. Repeat bolus if the pulse rate and capillary refill rate do not improve, but rarely is more than 20 mL per kg required in the first hour. The goal of this initial rehydration therapy is not euvolemia but adequate perfusion of end organs, often best judged by monitoring mentation, capillary refill, and heart rate.
Once adequate intravascular volume is established, the fluid deficit should be replaced over the next 48 hours. During the first 4 to 6 hours of this period isotonic fluids should be used with appropriate additional electrolyte supplementation as detailed below. The total body water deficit may be estimated based on a clinical estimate of dehydration, or intravenous (IV) fluid may be administered at a rate between one and one-half and two times maintenance fluid requirements (see Chapters 17 Dehydration and 108 Renal and Electrolyte Emergencies). Urine output should be monitored and ongoing urinary losses in excess of 5 mL/kg/hr (osmotic diuresis) should also be replaced.
The Na+ deficit typically approximates 10 mEq per kg body weight and Na+ maintenance is 3 mEq per 100 mL of maintenance fluid. From a practical point of view, half-normal (0.45%) saline can be started after the initial 4- to 6-hour period of isotonic fluids. The measured serum sodium should rise with initiation of therapy. If the initial serum sodium is less than 136 mEq per L, or if the serum sodium falls with therapy, the IV fluid should be changed to a more concentrated sodium stock, and the patient should be watched particularly closely. Serum sodium failing to rise with therapy has been identified as a risk factor for cerebral edema. Correcting the serum sodium for the degree of hyperglycemia may be useful in following the patient’s total body sodium status:
Corrected [Na+] = measured [Na+] + [2 × (measured plasma glucose − 100)/100].
All children with DKA are total-body potassium depleted (5 mEq per kg body weight); therefore, potassium replacement is an important part of therapy. If the initial serum [K+] is 3 to 4.5 mEq per L, 40 mEq per L of potassium is added to the infusion after vascular competency has been established and the child has urinated. If the serum [K+] is 4.6 to 5.0 mEq per L, only 20 mEq per L of potassium should be added, and if the [K+] is above 5.0 mEq per L, potassium should be withheld in the initial fluids. Generally, K+ is provided as potassium acetate (or chloride) and potassium phosphate in equal amounts. If the initial serum [K+] is less than 4 mEq per L, potassium replacement should be initiated promptly; if less than 3 mEq per L, IVF concentrations of K+ of 60 mEq per L or greater may be necessary. With the higher concentrations of potassium, the phosphate component must be adjusted not to exceed the maximum rate. If the K+ initial concentration is low, monitoring via an electrocardiogram (EKG) is indicated.
Phosphate depletion is almost universal in patients with DKA; however, the clinical significance of this reaction remains uncertain. As noted earlier, half of the K+ replacement is with potassium phosphate, up to a maximum of 20 mEq potassium phosphate per liter except in the rare situation of severe hypophosphatemia (serum phosphate less than 2 mEq per L). Infusion of excess phosphate results in hypocalcemia, which may be complicated by tetanic seizures.
Bicarbonate Therapy
In retrospective reviews of patients with DKA who developed significant cerebral edema, bicarbonate administration was identified as a significant risk factor (Table 97.3). This may be because the sickest patients are the ones most likely to have received bicarbonate therapy; however, without further clarification of the pathophysiology, bicarbonate therapy is reserved for patients with both severe acidosis (pH <6.9) and secondary hemodynamic compromise that is unresponsive to inotropic agents.
A theoretical mechanism for the complications observed with bicarbonate therapy is the development of a paradoxical acidosis of the central nervous system (CNS) and resultant cerebral depression. Paradoxical acidosis occurs because administered HCO3− combines with excess H+ ions in the bloodstream to form H2O and CO2. Because the blood–brain barrier is relatively more permeable to CO2 than to HCO3−, CO2 accumulates in the CNS, resulting in further exacerbation of acidosis in this compartment, while acidosis is being corrected systemically.
Insulin and Glucose
Regular insulin is used for the treatment of ketoacidosis, but it should not be administered until the initial isotonic fluids have been administered for 1 hour. Insulin is initially necessary to stop ongoing ketone body production, the primary cause of the acidosis. Insulin should be started after 1 hour of initial fluid expansion to steadily correct the acidosis and may be either infused intravenously or, if necessary, injected intramuscularly at hourly intervals. Subcutaneous injections of insulin should be avoided because of the uncertainties of absorption in a dehydrated patient. The starting dose of insulin for continuous infusion is 0.1 Unit/kg/hr, infused by a regulated pump. Failure of the glucose to decrease in response to insulin suggests improper insulin preparation, inadequate hydration, or serious underlying disease (e.g., appendicitis or fasciitis with resultant significant increases in counterregulatory hormones). It is unnecessary and possibly detrimental to give an initial bolus of insulin. The dose for the hourly intramuscular (IM) injection, used if IV access cannot be obtained, is 0.1 Unit/kg/hr.
Once the blood glucose approaches 300 mg per dL, dextrose should be added to the IV fluids. As long as the child remains acidotic, insulin infusion should never be stopped; instead, the amount of dextrose in the IV infusion should be increased in stepwise fashion up to a concentration of 12.5 g per dL to maintain the blood glucose between 200 and 300 mg per dL. If the blood glucose continues to drop, the rate of IV fluid administration should be increased to twice maintenance. If the blood glucose still cannot be maintained, the insulin infusion should be decreased by increments of 0.025 Unit/kg/hr.
When the child is able to eat and the anion gap has closed (normal = 10 to 12), IV infusion of insulin can be discontinued. If hourly IM injections are used, they should be continued until the blood glucose is less than 300 mg per dL and acidosis is correcting. Because IV insulin is metabolized rapidly, subcutaneous insulin must be given 30 minutes prior to the discontinuation of the infusion. The initial dose of subcutaneous insulin should be calculated, with consultation by a pediatric endocrinologist, based on a daily dose of 0.75 Unit/kg/day in the prepubertal child up to 1.0 Unit/kg/day in the pubertal child and beyond. The total daily dose must be divided into long-acting and short-acting insulins.
Cerebral Edema
Despite several investigations of the causes and risk factors for clinically significant cerebral edema in patients with DKA, and subsequent modifications in therapy, the incidence of the complication has not changed significantly during the past 20 years and remains at approximately 1%. Table 97.3 lists the leading risk factors published in more recent years. Clinical signs and symptoms of significant cerebral edema include abnormal motor or verbal response to pain, decorticate or decerebrate posturing, new cranial nerve palsy, and abnormal respiratory pattern. Other concerning signs are decrease or fluctuation in level of consciousness (e.g., Glasgow Coma Scale), age-inappropriate incontinence, vomiting, headache, and heart rate deceleration.
If these signs are noted by the physician at the bedside, a clinical diagnosis of cerebral edema must be made and treatment initiated without any diagnostic imaging. The patient should receive mannitol 1 g per kg IV over 10 minutes. There is some evidence that mannitol is the preferred first-line agent, but that hypertonic saline (3%) may be an appropriate second-line agent; however, only a large retrospective study and case series data are currently available.
Endotracheal intubation should be rarely considered, primarily if the patient’s mental status does not assure a safe airway, and secondarily if the patient is not able to maintain a respiratory alkalosis to partially compensate for the metabolic acidosis. Noninvasive ventilation may also be considered in order to support the patient’s efforts to achieve a respiratory alkalosis. If intubated, the patient should be initially hyperventilated to the PCO2 he/she was maintaining prior to the neurologic decompensation (generally 10 to 20 mm Hg in the presence of severe ketoacidosis); this can be gradually reduced over several hours as the acidosis resolves and the cerebral edema is treated.
Only after the patient is fully stabilized should a confirmatory computed tomography of the head be considered, unless a diagnosis of intracerebral hemorrhage or thrombosis is strongly suspected.
Clinical Indications for Discharge or Admission
Close monitoring is mandatory, and a well-organized flowsheet ensures all parameters are being observed. Admission to an intensive care unit or specialized intermediate care unit should be considered if the patient is younger than 1 year of age, has a Glasgow Coma Scale score of less than 12, has a venous pH below 7.1, has an initial measured [Na+] of more than 145 mEq per L, or has an initial [K+] of less than 3 mEq per L.
The patient should be maintained on continuous cardiorespiratory monitoring with hourly assessments of blood pressure and level of consciousness until the patient’s trajectory of illness has been clearly established. Careful neurologic examination, with particular attention to arousability and pupillary reactivity, should be performed frequently. The fluid input and output must be reviewed hourly to ensure appropriate rehydration is occurring. The IV fluids should be checked frequently so that pump failure or fluid leakage into the subcutaneous tissues can be corrected quickly. In the severely ill child, an EKG should be performed in the setting of hyperkalemia or hypokalemia. The plasma glucose should be measured hourly until the blood glucose is stable and less than 300 mg per dL, and as long as the child is on an insulin infusion. Glucose measurement may be less frequent once the patient has been changed to subcutaneous insulin. Serum [K+] needs to be measured every 2 to 4 hours until the acidosis and hyperglycemia are normalized, or more frequently if hypokalemia is encountered or bicarbonate therapy is used. Calcium, phosphate, and magnesium should be assessed initially and followed every 2 to 4 hours, more frequently if any are being actively replaced. With the advent of point-of-care ketone measurements, it may be advisable to follow serum ketone concentration every 2 to 4 hours, although continuous noninvasive capnography with nasal cannula end-tidal CO2 (ETCO2) or transcutaneous CO2 monitoring is also useful in tracking the degree of acidosis over time. Venous pH may be obtained to follow resolution of the acidosis if the above monitoring options are not available. Arterial sampling is not necessary for metabolic monitoring, and central venous access is rarely necessary.
When the child is better hydrated and the acidosis resolves, mental alertness will improve and symptoms of nausea, vomiting, and abdominal pain should remit. If they do not resolve, an abdominal disorder should be considered. Some patients complain of blurred vision, which is caused by lens distortion resulting from fluid shifts of rehydration and correction of hyperglycemia—this should resolve within 24 hours of conclusion of therapy. When the anion gap has closed, most patients are able to tolerate oral fluids, at which point rehydration can be continued orally ad libitum.
MILD KETOACIDOSIS/HYPERGLYCEMIA
Goals of Treatment
To identify patients with hyperglycemia and/or mild ketoacidosis and initiate treatment per algorithm.
To create a sick day plan for patients able to orally rehydrate, create sick day plan for them upon discharge with close follow-up with their diabetes specialist.
CLINICAL PEARLS AND PITFALLS
• Fasting laboratory plasma glucose of greater than 126 mg per dL or a random glucose greater than 200 mg per dL on two separate occasions is diagnostic of diabetes in an otherwise healthy person. This definition was developed by specialists in adult diabetes and may not be completely applicable to the pediatric population.
• Hyperglycemia in ED setting can result from numerous triggers including intercurrent illness or trauma in patient with known DM, new-onset DM, other illnesses associated with hyperglycemia, spurious blood sample, and medication effect.
• For purposes of definition, a patient with hyperglycemia does not have DKA if venous pH is greater than 7.3 and serum bicarbonate is greater than 15 mEq per L.
Current Evidence
As noted in the previous section on diabetes and the following section on hypoglycemia, glucose homeostasis reflects the balance between glucose input (from gut absorption, hepatic glycogen breakdown, or gluconeogenesis) and disposal (via storage or oxidation). With the exception of gut absorption, this process is largely regulated by insulin, although counterregulatory hormones also have a significant effect. Furthermore, tissue factors and medication also impact the insulin effect.
Clinical Considerations
Clinical Recognition
Plasma glucose concentrations in the 200 to 300 mg per dL range rarely result in symptoms. This level of hyperglycemia may be accompanied by intermittent increased frequency of urination; however, parents are rarely aware of their child’s frequency of urination once the child is toilet trained unless the frequency becomes disruptive (e.g., nocturia or “accidents” at school). Children and adolescents have no sense of what is the normal frequency of urination, so they rarely complain unless the frequent urination is accompanied by dysuria. Higher levels of glucose (greater than 300 mg per dL) may be associated with subtle clinical findings, such as blurring of vision or dryness of oral membranes. Significant hyperglycemia may occur without significant symptoms and can be tolerated for a prolonged period without clinical signs.
Triage
Generally these patients are asymptomatic and very well appearing. Care must be taken to distinguish from patients with more severe diabetic ketoacidosis and possible cerebral edema.
Initial Assessment/H&P
In the ED, hyperglycemia is likely to be seen in several different situations. First, the child may be known to have diabetes and present with an intercurrent illness or traumatic injury. Both illness and injury result in increased counterregulatory hormones, which may lead to relative insulin resistance and hyperglycemia. The second presentation is the child for whom diabetes is suspected because of classical symptoms of polyuria, polydipsia, and polyphagia accompanied by weight loss. Almost half of children with new-onset diabetes mellitus present to their pediatrician or to the ED in this way. Third, some medical conditions are associated with persistent hyperglycemia, such as recurrent urinary tract infections and vaginal yeast infections. Furthermore, type 2 diabetes is increasingly being reported in minority adolescents; in many, hyperpigmentation of the posterior neck and axilla (acanthosis nigricans) may be noted. Fourth, a laboratory panel obtained for some other reason (e.g., abdominal pain) may reveal hyperglycemia.
If a child is severely ill and has concomitant hyperglycemia, close attention should be paid to the underlying illness. Severity of hyperglycemia in the setting of critical illness is correlated with mortality, and it can be thought of as a general index of severity of illness in this nondiabetes setting.
Management/Diagnostic Testing
Children who are mildly dehydrated (5%) with slight acidosis will benefit from an IV fluid bolus (10 to 20 mL per kg of isotonic crystalloid); furthermore, this bolus may be given while awaiting laboratory test results.
Insulin therapy can be initiated subcutaneously, at a total daily dose of 0.25 to 0.5 Unit/kg/day for the prepubertal child and 0.5 to 0.75 Unit/kg/day for the adolescent. There are two general regimens for dividing this total daily dose. In the conventional regimen, two-thirds of the total daily dose is administered in the morning, and one-third before dinner; two-thirds of the morning dose and evening dose should be as an intermediate-duration insulin (NPH, Lente), the remaining one-third of the total daily dose is rapid-acting insulin (lispro, aspart). Using the basal-bolus approach, one-half of the total daily dose is administered as insulin glargine or detemir, two 24-hour–acting analogs, and rapid-acting insulin (lispro, aspart) is dosed as a combination of coverage for ingested carbohydrates and as a correction for the degree of hyperglycemia above a chosen target—these initial dosages should be calculated along with the help of a consulting diabetes specialist.
Hyperglycemia associated with critical illness should be managed in the context of the underlying illness. Specific therapy for hyperglycemia should generally not be initiated in the ED, but can wait until the patient arrives in the ICU.
Clinical Indications for Discharge or Admission
Some children with new-onset diabetes may also have hyperglycemia without ketoacidosis or with only mild acidosis. Generally, these patients are hospitalized for 24 to 48 hours to allow time to educate the family and stabilize the insulin dosage. Children with known diabetes often develop hyperglycemia and ketosis without significant acidosis (venous pH greater than 7.3 or bicarbonate greater than 15 mEq per L) during the course of intercurrent illness, especially gastroenteritis, or secondary to omission of insulin doses. Once the laboratory results are available, the physician must decide whether to hospitalize the child, continue treatment in the ED, or send the child home. Several factors must be considered before sending a child home.
1. Is the child fully conscious and alert?
2. Can the child drink and retain oral fluids?
3. Can home glucose monitoring be done and are all related supplies available in the home?
4. Can ketones be measured at home, either in the urine with chemical test strips or in the serum with a point-of-care blood measurement device?
5. Will the child have competent supervision at home?
6. Does the family have access to both a telephone and transportation?
7. Is there a clinician available with whom the family can communicate by telephone?
8. Is the family comfortable with managing the mild acidosis at home?
If these questions can be answered in the affirmative, the child may be sent home. Recommendations should be made to the family regarding fluid intake, insulin administration, and monitoring. Specific recommendations may vary with the age of the child and the experience of the family, but the following scheme may be helpful. Oral intake should be about the same as would be given intravenously to resolve the deficit and provide maintenance (e.g., the 10-year-old child [30 kg] would normally receive a 300-mL bolus followed by 100 to 140 mL per hour, for a total of up to 1 L during the first 6 hours intravenously if he/she was hospitalized; therefore, the physician should suggest that the family try to get in 150 to 180 mL of liquid every hour for the next 6 hours). It is best if this liquid is taken in as sips. Supplements of short-acting insulin will be required in addition to the patient’s usual long-acting doses. In the ED, two decisions will need to be made regarding insulin.
 First, how much short-acting insulin (lispro or regular) should be given to the child before discharge? One way to dose additional insulin is using the 5–10% to 10–15% rule.
First, how much short-acting insulin (lispro or regular) should be given to the child before discharge? One way to dose additional insulin is using the 5–10% to 10–15% rule.
 If blood glucose is 250 to 400 mg per dL without urinary ketones, 5% of the child’s usual total daily dose will suffice.
If blood glucose is 250 to 400 mg per dL without urinary ketones, 5% of the child’s usual total daily dose will suffice.
 If blood glucose is more than 400 mg per dL without ketones, or is 250 to 400 mg per dL with moderate or large ketones, 10% of the daily dose will be needed.
If blood glucose is more than 400 mg per dL without ketones, or is 250 to 400 mg per dL with moderate or large ketones, 10% of the daily dose will be needed.
 If blood glucose is more than 400 mg per dL and ketones are moderate or large, the child will need 15% of the daily dose and admission to the hospital should be reconsidered.
If blood glucose is more than 400 mg per dL and ketones are moderate or large, the child will need 15% of the daily dose and admission to the hospital should be reconsidered.
 Second, how much insulin should be given at home and with what frequency? Once home, the preceding 5–10% to 10–15% rule is generally applicable and should be given every 4 hours, based on blood glucose and blood or urinary ketones. The family can begin using this algorithm once the child is able to return to a normal intake. For any child to be safely discharged home, however, he or she must be able to maintain adequate oral intake and have frequent contact with a clinician who is comfortable managing pediatric diabetes. Finally, hourly monitoring of blood glucose, urine output, and ketones is recommended with the expectation that the blood glucose should decline, the urine output should fall, and the ketones should begin to clear.
Second, how much insulin should be given at home and with what frequency? Once home, the preceding 5–10% to 10–15% rule is generally applicable and should be given every 4 hours, based on blood glucose and blood or urinary ketones. The family can begin using this algorithm once the child is able to return to a normal intake. For any child to be safely discharged home, however, he or she must be able to maintain adequate oral intake and have frequent contact with a clinician who is comfortable managing pediatric diabetes. Finally, hourly monitoring of blood glucose, urine output, and ketones is recommended with the expectation that the blood glucose should decline, the urine output should fall, and the ketones should begin to clear.
Failure to respond to these simple measures, whether in the ED or at home, should lead to a consultation with the child’s endocrinologist. If oral fluids must be restricted and the child is hyperglycemic (e.g., a child with traumatic injury requiring surgery), IV fluids without glucose should be used and glucose should be monitored frequently. As blood glucose concentration reaches 200 mg per dL, dextrose should be added to the IV fluid to maintain target blood glucose of 150 to 250 mg per dL. Additional supplemental insulin may be required, depending on when the child last received insulin and the response to simple hydration. Note, if hyperglycemia is a coincidental finding, the diagnosis requires thoughtful consideration. How traumatic was the blood draw? How upset was the child? What medications or IV fluids were given to the child just before the phlebotomy? What was the child drinking while waiting to see the physician? Are the symptoms in any way related to the hyperglycemia? How sick is the child? The sicker the child is, the less likely it is that hyperglycemia is reflective of diabetes. Three simple evaluations are helpful in determining whether the hyperglycemia is circumstantial or suggestive of diabetes. Brief hyperglycemia resulting from a stress response to phlebotomy or secondary to oral intake rarely results in significant glucosuria; therefore, a urine dip for glucose is often helpful. Second, in the absence of ongoing stress or input, glucose tends to fall over time. A point-of-care glucose is rarely stressful. Therefore, repeating a glucose measurement by fingerstick 1 to 2 hours after the original sample was sent is useful in separating disease from nondisease. Third, hyperglycemia secondary to these factors is usually mild (150 to 250 mg per dL). More significant hyperglycemia should raise the suspicion of diabetes, glucose intolerance, or an underlying medical illness that is producing a significant counterregulatory response.
HYPOGLYCEMIA
Goal of Treatment
To recognize hypoglycemia, initiate a diagnostic laboratory evaluation, and begin corrective treatment immediately if exhibiting any symptoms.
CLINICAL PEARLS AND PITFALLS
• Hypoglycemia in absence of ketones is consistent with hyperinsulinism or fatty acid oxidation enzyme deficiencies.
• Every acutely ill child with an altered level of consciousness should have a rapid bedside glucose determined.
• Treat severe hypoglycemia with rapid IV administration of 0.25 g dextrose per kg body weight.
Current Evidence
Hypoglycemia is generally defined as plasma glucose of less than 50 mg per dL, regardless of whether symptoms are present. A differential diagnosis of hypoglycemia, as it may present in the ED, is provided in Table 97.4. Hypoglycemia may be secondary to insulin therapy for diabetes. Excluding this category, almost all hypoglycemia in children occurs during periods of decreased or absent oral intake, often coupled with increased energy demand (e.g., viral gastroenteritis with fever). Postprandial hypoglycemia is unusual in children, except in those who have had prior gastrointestinal surgery. A few select poisonings can produce hypoglycemia. Because glucose is necessary for cellular energy production in most human tissues, the maintenance of an adequate blood glucose concentration is important for normal function. The plasma glucose reflects a dynamic balance among glucose input from dietary sources, glycogenolysis and gluconeogenesis, and glucose use by muscle, heart, adipose tissue, brain, and blood elements. The liver plays a unique role in glucose homeostasis because it stores glucose as glycogen. With fasting, this glycogen is degraded to glucose, which is released into the bloodstream. In addition, the liver synthesizes new glucose from glycerol, lactate, and certain amino acids. During fasting, lipolysis occurs and the resultant fatty acids are used for the production of both energy and ketones (acetoacetate and β-hydroxybutyrate) by the liver. The energy generated from the metabolism of fatty acids is essential to sustain maximal rates of gluconeogenesis and ureagenesis in the liver. The ketones are an important auxiliary fuel for most tissues, including the brain. Muscle contains significant quantities of glycogen and protein. Under fasting conditions, the glycogen is degraded and used endogenously but is not released as free glucose into the bloodstream. Certain amino acids, particularly alanine and glycine, are released from the muscle and subsequently used by the liver for gluconeogenesis. Muscle derives an increasing proportion of its energy requirement from fatty acids as fasting proceeds. Brain tissue is highly dependent on glucose for its energy requirements. Under certain circumstances, it can extract a limited proportion of its energy requirement from other substrates (e.g., glycerol, ketones, lactate), although this process requires a period of adaptation and does not obviate the need for a constant supply of glucose. Insulin is the primary hormone that regulates the blood glucose level. Insulin stimulates the uptake of glucose and amino acids into skeletal, cardiac, and adipose tissue and promotes glycogen and protein synthesis. It inhibits lipolysis and glycogenolysis. The net effect of insulin action is to accelerate the removal of glucose and gluconeogenic substrates from the bloodstream. Opposing or modulating the effects of insulin are cortisol, glucagon, epinephrine, and growth hormone. The effects of these hormones include inhibition of glucose uptake by muscle, mobilization of amino acids for gluconeogenesis, activation of lipolysis, inhibition of insulin secretion, and induction of gluconeogenic enzymes. The net effect is to increase the availability of gluconeogenic substrates to the liver, and to increase the accessibility and use of nonglucose fuels by other tissues.
TABLE 97.4
CAUSES OF CHILDHOOD HYPOGLYCEMIA

Clinical Considerations
Clinical Recognition
The acutely ill child warrants a glucose determination if the level of consciousness is altered because hypoglycemia may accompany an illness that interferes with oral intake. The symptoms and signs of hypoglycemia are nonspecific and are often overlooked, especially in the infant and young child. Any child presenting with a seizure, other than a breakthrough seizure with known epilepsy, or unconsciousness should have a plasma glucose determination.
Triage
Children with known diabetes who appear ill need a rapid bedside glucose for possibility of hypoglycemia or hyperglycemia. All children with acute alterations in consciousness, including those with dehydration and fussy or lethargic young infants, should have a point-of-care glucose measurement.
Initial Assessment/H&P
Because hypoglycemia in children occurs after a period of fasting, a careful chronology of dietary intake during the preceding 24 hours should be obtained, as well as a history either of poor fasting tolerance (irritable upon awakening until feeding), or of fasting avoidance (sleeps with bottle in crib). The possibility of a toxic ingestion should be considered because ethanol, β-blockers, and oral hypoglycemic agents are in common use. Family history should be explored for evidence of an undiagnosed metabolic disorder.
The clinical findings of hypoglycemia reflect both the decreased availability of glucose to the CNS and the adrenergic stimulation caused by decreasing or low blood glucose. Adrenergic symptoms and signs include palpitations, anxiety, tremulousness, hunger, and sweating. Irritability, headache, fatigue, confusion, seizure, and unconsciousness are neuroglycopenic symptoms. Any combination of these symptoms should lead to a consideration of hypoglycemia.
Management/Diagnostic Testing
If hypoglycemia is suspected, blood should be drawn before treatment, if at all possible. An extra tube (3 mL serum) should be obtained and refrigerated until the laboratory glucose is known. Rapid screening should be performed using a bedside glucose meter while awaiting definitive laboratory results. In some clinical laboratories, blood glucose can be emergently obtained with heparinized “whole” blood samples along with blood gases. Therapy should be instituted if this screen is suggestive of hypoglycemia. This method may lead to some overtreatment because of error of bedside devices; however, treatment holds minimal risk. It is preferable to overtreat than to allow a child to remain hypoglycemic until definitive laboratory results are available. If the laboratory glucose confirms that the blood glucose was less than 50 mg per dL, the reserved serum can be used for chemical (β-hydroxybutyrate, acetoacetate, amino acid profile, acylcarnitine profile), toxicologic, and hormonal (insulin, growth hormone, cortisol) studies, and may provide the correct diagnosis without extensive additional testing. If adequate blood is obtained before correction, other metabolites to be considered are glucagon, C-peptide, lactate, and pyruvate. If blood is obtained with 15 minutes of glucose administration, it may still be helpful, although possibly not diagnostic. The first voided urine after the hypoglycemic episode should be saved for toxicologic, organic acid evaluation, and acylglycine profile. In the ED, the urine should also be tested immediately for ketones. With hypoglycemia, ketones should be present. Failure to find moderate or large ketone concentrations in the presence of hypoglycemia strongly suggests either that fats are not being mobilized from adipose tissue, as might occur in hyperinsulinism, or that fat cannot be used for ketone body formation, as might occur in enzymatic defects in fatty acid oxidation (e.g., medium chain acyl dehydrogenase [MCAD] deficiency, and many other metabolic defects—see Chapter 103 Metabolic Emergencies). Both the urine and the serum results will be useful in determining the underlying cause of hypoglycemia.
The preferred treatment for hypoglycemia is rapid IV administration of 0.25 g of dextrose per kg body weight (2.5 mL per kg of 10% dextrose, 1.0 mL per kg of 25% dextrose). The plasma glucose should then be maintained by an infusion of dextrose at a rate of 6 to 8 mg/kg/min. Generally, this goal can be accomplished by providing 10% dextrose at one and one-half times maintenance rates. While waiting for vascular access, mucosal and enteral routes should be considered if can be done safely. Glucagon (0.03 mg per kg up to a maximum of 1 mg intramuscularly) may be used to treat hypoglycemia that is known to be caused by hyperinsulinism but is not indicated as part of the routine therapy of hypoglycemia. Glucocorticoids should not be used because they have minimal acute benefit and may delay identification of the cause of hypoglycemia.
The adequacy of therapy should be evaluated both chemically and clinically. The plasma glucose should be monitored frequently and consistently until a stable level higher than 70 mg per dL is attained on more than one measurement. Adrenergic symptoms should resolve quickly. The resolution of CNS symptoms may be prolonged, particularly if the child was initially seizing or unconscious. Seizures that do not respond to correction of hypoglycemia should be managed with appropriate anticonvulsants (see Chapters 67 Seizures and 105 Neurologic Emergencies). The mild acidosis (pH 7.25 to 7.35) usually seen in hypoglycemia will correct without specific intervention. Marked acidosis (pH <7.10) suggests shock or serious underlying disease and should be managed appropriately (see Chapter 5 Shock).
Clinical Indications for Discharge or Admission
Any child with documented hypoglycemia not secondary to insulin therapy, or due to another known entity, should be considered for hospitalization for careful monitoring and diagnostic testing. Exceptions to hospitalization might include children with significant dehydration in the setting of a gastroenteritis illness where symptoms are improving or controlled after proper rehydration in the ED. If being considered for discharge, these children will need repeat blood glucose measurements off IV infusions for several hours prior to discharge.
HYPOPITUITARISM
Goal of Treatment
The goal of treatment in those with known hypopituitarism includes the replacement of essential hormones especially during times of stress such as illness or injury.
CLINICAL PEARLS AND PITFALLS
• The acute presentation of hypopituitarism is most likely to occur when the child is stressed by injury, illness, or fasting.
• Children with midline neurologic defects are at risk for hypopituitarism.
• Children with hypopituitarism are prone to hypoglycemia.
• Hypopituitarism is associated with intracranial lesions.
• Cortisol replacement in patients with adrenal insufficiency under stress conditions is imperative.
Current Evidence
The term hypopituitarism
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







