2. Oral hypoglycemic agents
a. Women with pregestational type 2 DM who require oral agents for glucose control should ideally be changed to insulin before pregnancy. Although there is no evidence to suggest that metformin and glyburide are teratogenic, the marked increase in insulin resistance during pregnancy usually results in women with type 2 DM requiring insulin for glycemic control and potential reduction of perinatal mortality.19 Data on the long-term effects of meglitinides, thiazolidinediones, dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) analogues, and sodium-glucose co-transporter-2 (SGLT-2) antagonists are lacking in pregnancy.
b. Most clinicians prefer to find an optimal insulin regimen before pregnancy to avoid unstable glycemic control during organogenesis. Oral hypoglycemic agents should not be discontinued in women who conceive while receiving oral hypoglycemic agents, until insulin therapy is initiated as hyperglycemia is potentially much more teratogenic than any of the currently available oral therapies used to treat DM. Current data has shown that the use of oral diabetic medications during pregnancy has not resulted in harmful, short-term effects, but evidence is lacking on the effects of long-term use.1
c. GDM is a milder metabolic derangement more easily controlled by oral hypoglycemic agents such as glyburide and metformin. Although the U.S. Food and Drug Administration has not approved these oral agents for treatment of GDM, current evidence from several trials have demonstrated good glycemic control with glyburide or metformin, although there is some concern for an increased risk for hypoglycemia with glyburide use; up to 50% of women will need insulin supplementation as well. Data do not show an increased risk of unfavorable short-term maternal or neonatal outcomes with the use of glyburide or metformin.20,21 Although glyburide has been reported to cross the placenta in one study, another study was unable to detect glyburide levels in umbilical cord blood analyses.2 Thus, women with GDM should be counselled on the risks versus benefits of oral hypoglycemic agents versus insulin for glycemic control when diet modification alone is insufficient.
3. Diabetic ketoacidosis in pregnancy
a. DKA is the triad of hyperglycemia, ketosis, and metabolic acidosis that is usually accompanied by significant intravascular volume and total body potassium depletion. It most often occurs in women with type I diabetes. The metabolic changes that accompany pregnancy make the pregnant woman more prone to DKA and include (i) insulin resistance, (ii) accelerated starvation, (iii) hyperemesis-associated dehydration, and (iv) need to compensate for a progesterone-induced respiratory alkalosis with renal excretion of bicarbonate.22 DKA is most common in second and third trimesters. Precipitating events for DKA include omission of insulin in insulin requiring pregnancies (often an inappropriate reaction to hyperemesis), infection, and corticosteroid use to promote lung maturity. DKA may also occur in type 2 DM, albeit rarely, especially in the African American population. Women with type 2 diabetes may be more likely to develop a hyperosmolar hyperglycemic nonketotic state (HHNS) portrayed by hyperglycemia, serum hyperosmolality (>360 mOsm per L), and severe hypovolemia without ketonemia.23
b. The presentation of DKA is similar in nonpregnant and pregnant patients, although the glucose levels may be much lower than in nonpregnant patients with DKA.22 The physiologic respiratory alkalosis of pregnancy results in lowered buffering capacity allowing more rapid changes in pH, and the high GFR in pregnancy causes continued renal excretion of glucose. Therefore, acidosis is more pronounced at lower serum glucose levels.22
c. DKA in pregnancy is a medical emergency that requires immediate treatment. A high fetal mortality rate is associated with DKA, although it has decreased from 27% (from 1950 to 1979) to 9% (from 1985 to 1995).22 Treatment requires prompt recognition, maternal stabilization, rehydration, intravenous (IV) insulin therapy, and electrolyte replacement as in the nonpregnant patient. Precipitating factors should be investigated and treated. Continuous fetal monitoring and full fetal assessment should be utilized. Urgent cesarean delivery (CD) while the mother is still acidotic is not recommended due to higher maternal risk with minimal benefit to the fetus. Fetal compromise may improve once the maternal metabolic condition reverses.
CLINICAL PEARLThe incidence of DKA in women with DM prior to pregnancy is 5% to 10%. DKA can be present with only mildly increased glucose levels in pregnant women; thus, the diagnosis requires a high index of suspicion.
4. Assessment and surveillance for complications
a. Renal disease. Women with known nephropathy must be closely followed for increasing proteinuria, renal insufficiency, and hypertension. It is very difficult, and at times impossible, to differentiate worsening nephropathy from superimposed preeclampsia. A microalbumin per creatinine ratio or 24-hour urine collection for creatinine clearance (CrCl) and protein excretion (including microalbumin levels) should be obtained in each trimester and more often if progression is seen. The mean increase in protein excretion is approximately 3 g per 24 hours throughout gestation.24 Microalbuminuria usually increases but rarely to the level of overt nephropathy. Postpartum renal function will return to prepregnancy levels in most cases.6 For women with moderate-to-severe renal impairment (serum creatinine level >124 μmol per L or CrCl <70 mL per minute) before conception, there is a 45% risk of pregnancy-related permanent decline in GFR.7 Hypertension is seen in 30% of women with diabetic nephropathy in the first trimester and in 75% by the third trimester. Deteriorating renal function and superimposed preeclampsia are responsible for the high rates of preterm delivery (>50%), low birth weight, and CD.
b. Retinopathy. The baseline severity of retinopathy is highly predictive of its progression during pregnancy. Frequent ophthalmology assessments are necessary throughout gestation, if significant retinopathy is present. Many practitioners recommend an assisted vaginal delivery due to the increased risk of retinal bleeding; however, there is little evidence to support this recommendation.
E. Management during labor and delivery
1. Timing of delivery
Optimal timing of delivery for the woman with DM requires balancing the risks of prematurity with the risks of worsening maternal and fetal health. Patients with well-controlled DM, in the absence of worsening complications and no evidence of nonreassuring fetal status, may go to their expected date of delivery but generally not past 40 weeks.15 A pregnancy complicated by macrosomia is at greater risk for maternal and infant birth trauma, which may be an indication for earlier delivery with an estimated fetal weight of >4,500 g. However, the indications for induction for vaginal delivery and elective CD in pregnancies complicated by macrosomia are controversial, and outcome studies are sparse.25 Decreasing insulin requirements in late gestation, not related to a decrease in carbohydrate intake, may be a sign of placental failure. Although not well validated, many practitioners will proceed with delivery if there has been a significant decrease in insulin dosage unrelated to diet. Other indications for intervention in these women include worsening diabetic nephropathy, superimposed preeclampsia, and evidence of nonreassuring fetal status. Nonstress testing and Doppler measurements are often used to assess the fetal status.
2. Glucose management
Women with GDM will generally not require insulin during labor, whereas women with type 1 and type 2 will require careful monitoring and adjustment of insulin. The goals are to avoid maternal hyperglycemia and reduce risk of maternal and neonatal hypoglycemia while incorporating patient preference for autonomy of care. Maintaining the intrapartum maternal glucose level <120 mg per dL may reduce neonatal hypoglycemia26; however, many women will develop hypoglycemia. Changing oral intake, stress of labor, and the change in insulin sensitivity after placental delivery make glycemic control challenging. In general, subcutaneous (SQ) insulin can be continued until the woman is in active labor or is unable to eat. Then, IV insulin should be initiated with the dose adjusted based on hourly glucose monitoring. IV insulin protocols should be developed locally with input from all care providers to reduce risk of medication error. Women who use a continuous SQ insulin infusion (pump) can continue to use the pump throughout labor and delivery, provided they are willing to take responsibility for adjusting doses during delivery.
3. Anesthetic management
Anesthesiologists can expect to care for diabetic parturients in a variety of patient care settings including premature labor, uncomplicated labor and vaginal delivery, complicated obstetric disorders (e.g., preeclampsia), as well as elective or emergent CD. Maternal, fetal, and neonatal complications can be expected in this patient population; therefore, early preanesthetic evaluation is essential. This will include careful airway examination (e.g. to rule out stiff joint syndrome), assessment of blood glucose control and renal function, as well as documentation of the presence or absence of peripheral neuropathy. This assessment is helpful in the detection of the chronic complications of diabetes due to microvascular disease.
a. Labor analgesia
Parenteral opioids can be used in early labor for pain control in uncomplicated diabetic patients, but early epidural analgesia offers many benefits. Labor epidural analgesia not only provides superior pain relief but also reduces maternal endogenous catecholamine levels, resulting in improved placental perfusion and reduced insulin requirements. Because many diabetic mothers are at increased risk for urgent or emergent CD, a functioning epidural catheter allows for rapid induction of anesthesia, and avoids the need for general anesthesia.
After noting baseline maternal vital signs and fetal heart rate (FHR), either epidural or combined spinal-epidural (CSE) analgesia can be administered safely. Although some practitioners prefer epidural analgesia because of the ability to titrate the block and confirm the block level, CSE techniques have been shown to be as reliable as epidural only techniques. Regardless of the technique, a well-conducted epidural or CSE should be administered with care, paying particular attention to maintenance of uterine displacement, avoiding hypotension and administration of non–dextrose-containing fluids. Hypotension unresponsive to IV fluids should be treated promptly with either ephedrine or phenylephrine because even minor degrees of hypotension may worsen uteroplacental insufficiency in diabetic parturients. Women with overt nephropathy are at risk for volume overload; therefore, IV fluid administration should be closely monitored.
b. Cesarean delivery
Anesthetic considerations for CD in the diabetic parturient are similar to those for labor analgesia: (i) avoid hypotension, (ii) administer non–dextrose-containing IV fluids, and (iii) maintain uterine displacement. Spinal, epidural, or general anesthesia can be administered safely but the chosen technique should be individualized based on several factors, including anesthetic, obstetric, or fetal risk factors (e.g., elective vs. emergency); the preferences of the patient; and the judgment of the anesthesiologist. Neuraxial techniques are preferred to general anesthesia for most CD. However, general anesthesia may be the most appropriate choice in some circumstances (e.g., profound fetal bradycardia, ruptured uterus, severe hemorrhage, and severe placental abruption).
CLINICAL PEARLAnesthetic management should focus on maintaining appropriate glucose control, modification based on the presence and severity of preexisting end organ disease, and maintaining an optimal fetal environment.
4. Postpartum care
a. Insulin requirements. Following delivery of the placenta, insulin sensitivity returns immediately. Maternal hypoglycemia should be avoided. A reduction of insulin dosage to two-thirds of the prepregnancy requirement is prudent. For women with type 1 DM, the first SQ dose of insulin should be administered before discontinuing an IV infusion due to the short half-life of IV insulin. For women on oral agents or diet before pregnancy, insulin should be used only if the patient clearly has postpartum hyperglycemia.
b. Neonatal hypoglycemia must be anticipated and local protocols established. In general, infants born to these mothers should have frequent capillary glucose measurements and, if the blood glucose level is <2 μmol per L (<36 mg per dL), early oral feeding or the use of IV dextrose should be implemented. Other neonatal complications include (a) hypocalcemia, (b) jaundice, (c) polycythemia, (d) septal hypertrophy, (e) sacral agenesis, and (f) respiratory distress syndrome.
CLINICAL PEARLThe risk for neonatal hypoglycemia following delivery may be minimized by good maternal glucose control during labor and vaginal and operative delivery.
II. Thyroid disorders
A. Introduction
Thyroid disorders are common in women of childbearing age with an incidence estimated at 4% to 5%. Women may (a) have previously diagnosed thyroid disease that requires monitoring during pregnancy, (b) have unrecognized thyroid disease that exacerbates during pregnancy, or (c) develop pregnancy-induced thyroid dysfunction during or after pregnancy. Depending on the underlying cause of the thyroid disorder and severity of change in thyroid hormone levels, there can be detrimental consequences for both maternal and fetal health.
B. Thyroid physiology
1. Pregnancy is associated with significant but reversible changes in maternal thyroid physiology. There are two forms of thyroid hormone—thyroxine (T4) and triiodothyronine (T3). Most circulating hormone is T4, of which only 0.04% is unbound (“free T4”) and physiologically active. The remainder is bound to circulating transport proteins. T4 is converted intracellularly to T3, which is required for in biologic action. Thyroid-stimulating hormone (TSH) is the primary regulator of thyroid function; it increases synthesis and release of thyroid hormone.
2. The pregnant woman makes 50% more thyroid hormone during pregnancy to compensate for increased levels of binding hormone and increased clearance of hormone through placental degradation. The fetal thyroid gland begins to synthesize thyroid hormone at 12 weeks’ gestation, but remains dependent on maternal thyroid hormone until the thyroid–pituitary axis begins functioning at 18 weeks.
3. There are normal, physiologic changes in thyroid hormone levels during pregnancy. The rising levels of human chorionic gonadotropin (hCG) in the first trimester result in a transient increase in T4 production and suppression of TSH. It is therefore “normal” to have a slightly suppressed TSH and slightly elevated free T4 during the first trimester (see subsequent text). TSH will return to normal nonpregnant levels once hCG levels fall. Most studies have demonstrated a mild decrease in free T4 and slight increase in TSH in the third trimester, usually within the normal range.
4. Most thyroid disorders are autoimmune in nature. The thyroid-stimulating hormone receptor antibodies (TSHrAbs) associated with Graves disease have been shown to decrease substantially during pregnancy, allowing discontinuation of medical treatment for many women. In those women who continue to have high antibody titers during pregnancy, passive placental transfer can lead to fetal thyroid disorders after 18 weeks. Antibody titers will increase postpartum and are responsible for flares of Graves disease and appearance of postpartum thyroiditis.27
5. Some, but not all, clinical practice guidelines/expert panels recommend routine thyroid screening preconceptually, or when pregnancy is identified, due to an increased risk for impaired brain development in children of mothers with abnormal thyroid function during pregnancy.28 Table 23.2 compares the overall changes in thyroid function tests in normal pregnancy versus thyroid disease states.
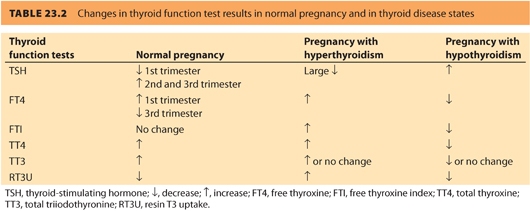
CLINICAL PEARLNormal pregnancy requires an increase in thyroid production by the maternal thyroid gland, which can be insufficient due to a variety of causes.
C. Hyperthyroidism
1. Definition and pathophysiology
Hyperthyroidism is defined by excessive thyroid hormone production. It can be subclinical (suppressed TSH, normal T4 and T3) or overt (suppressed TSH and elevated T4 and/or T3). Severe thyrotoxicosis is associated with poor obstetric outcomes. Women with uncontrolled hyperthyroidism have a fivefold greater risk of developing severe preeclampsia.27 There is also an increased risk of fetal loss, low birth weight, prematurity, and placental abruption, which appear to be directly related to the blood level of maternal T4. Women who are appropriately treated and euthyroid before pregnancy do not have increased risk for these poor outcomes.28
2. Physiology of hyperthyroidism
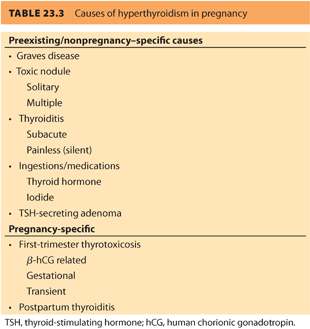
In the first trimester, 10% to 20% of normal women will have a suppressed TSH due to the effect of hCG. Some women, especially those with high hCG levels associated with hyperemesis, molar pregnancy, and twins will have gestational thyrotoxicosis, also referred to as first-trimester thyrotoxicosis and hCG-related thyrotoxicosis. This temporary condition improves as hCG levels decrease and does not usually require medical intervention. The most common cause of preexisting hyperthyroidism is Graves disease, an autoimmune hyperthyroidism secondary to stimulation of the thyroid gland by TSHrAb. Other causes include (a) toxic solitary nodule, (b) multiple nodular goiter, (c) ingestions (e.g., exogenous thyroid hormone, amiodarone, excess iodine), and (d) subacute thyroiditis (see Table 23.3).
3. Clinical presentation of hyperthyroidism
a. The clinical presentation of hyperthyroidism in pregnancy will depend on the underlying cause and severity of the thyrotoxicosis. All causes of thyrotoxicosis may present with adrenergic symptoms including (i) nervousness, (ii) heat intolerance, (iii) weight loss, (iv) diarrhea, (v) palpitations, (vi) anxiety, (vii) lid retraction, and (viii) stare. Features specific to the underlying cause may be detected on the thyroid examination. The diffuse, symmetric, soft goiter may have an audible bruit and may be a feature of Graves disease. Symptoms are often exacerbated in the first trimester of pregnancy and improve as the pregnancy advances.29 In the setting of subacute thyroiditis, there may be a palpable nodule associated with nodular disease (usually >3 cm) and tenderness.
b. Autoimmune manifestations such as (i) orbitopathy (proptosis, soft tissue periorbital swelling, and extraocular muscle dysfunction), (ii) pretibial myxedema, and (iii) clubbing may be present and are exclusive to Graves disease. If clinical findings are inconclusive, it is helpful to measure TSHrAb provided that the results are available in a timely manner; however, TSHrAbs are positive only in 80% of patients with Graves disease.30
4. Management
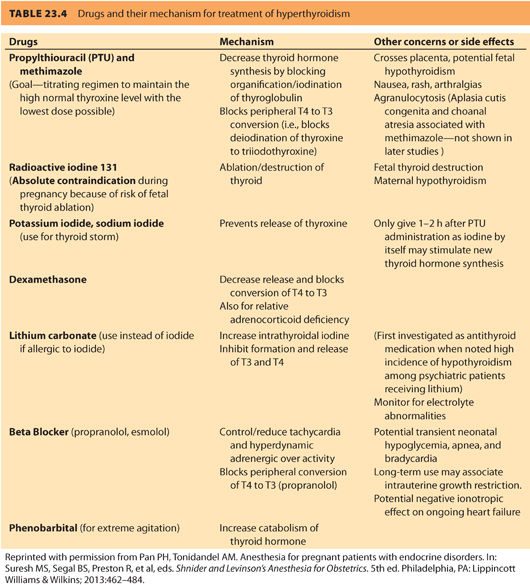
The goal of management in all cases of thyrotoxicosis is to normalize, but not suppress, thyroid hormone levels and to treat bothersome symptoms. In the nonpregnant state, three treatment options exist for hyperthyroidism: (a) antithyroid medication, (b) radioactive iodine to partially ablate the thyroid gland, and (c) near-total thyroidectomy. The biochemical status of women who have chosen radioactive iodine or surgery before pregnancy, no longer reflects their autoimmune status. Women with Graves disease who previously received ablative therapy (iodine 131 [131I] or thyroidectomy) have a continued risk of fetal Graves disease if the antibody titer is still significantly elevated. Propylthiouracil (PTU) and methimazole are both used as medical therapy for Graves disease. PTU is generally preferred due to concern over congenital anomalies reported with methimazole use.31,32
5. Fetal outcome
a. Fetal risk depends on the degree of thyrotoxicosis, the underlying cause of the thyrotoxicosis, and the treatment modality used. In most cases, the neonate of a hyperthyroid mother is euthyroid. Hyper- or hypothyroidism may occur and either condition can develop with or without a neonatal goiter. Neonatal hyper- and hypothyroidism that is secondary to maternal thyroid dysfunction is usually transient and will respond to therapy within 1 to 2 weeks.
b. Placental transfer of TSHrAb can cause excessive stimulation of the fetal thyroid gland resulting in fetal or neonatal Graves disease. This occurs after 16 weeks of gestation with (i) fetal tachycardia, (ii) high-output cardiac failure, (iii) hydrops, (iv) craniosynostosis, (v) IUGR, and (vi) fetal goiter. Fetal goiter can also be caused by placental transfer of maternally administered thioamides. In this situation, the fetus is hypothyroid.
c. Chronic exposure to hyperthyroidism from inadequately treated maternal hyperthyroidism may impair maturation of the fetal hypothalamic-pituitary-thyroid axis. This can lead to central congenital hypothyroidism in the infant.
CLINICAL PEARLThyrotoxicosis is associated with increased rates of spontaneous abortion, preeclampsia, and preterm delivery. Only Graves disease–related thyrotoxicosis is associated with neonatal Graves disease.
6. Antepartum considerations
a. Medications. The treatment goal is a free T4 level in the high normal range using the lowest dose of thioamide possible. For symptom relief, β-blockers can be used until the free T4 levels come into the normal range. Table 23.433 summarizes the common drugs and their action of mechanism for treatment of hyperthyroidism. Excessive use of antithyroid medication can result in fetal hypothyroidism and fetal goiter and must be avoided. Gestational thyrotoxicosis is self-limiting and does not require thioamide treatment. For individuals who develop adverse reactions to thioamides, especially significant leukopenia, or liver abnormalities, a thyroidectomy in pregnancy may be required.
b. Fetal goiter/Graves disease. In women medically treated for Graves disease, fetal goiter can occur from thioamide-induced fetal hypothyroidism or fetal Graves disease. Women who receive radioactive iodine or a thyroidectomy may continue to produce high titers of TSHrAb and place their fetuses at risk for Graves disease. For women not on antithyroid medication and who have negative TSHrAb, no additional fetal monitoring is required.34 Fetal monitoring should include assessment for tachycardia, fetal growth, and goiter size by ultrasonography starting at 20 weeks’ gestation and repeated monthly. If there is no evidence of fetal goiter, significant fetal thyroid dysfunction is unlikely. Rapid resolution of the fetal goiter is possible with in utero therapy.
7. Thyroid storm. Pregnancy is a potential rare cause of thyroid storm and is associated with a very high mortality rate of 25%.35 Diagnosis is by clinical detection of a rapid-onset, severe, life-threatening exacerbation of thyrotoxicosis that usually occurs in the presence of significant goiter. Other life-threatening illnesses (sepsis, hypoglycemia, pheochromocytoma, and cocaine toxicity) may mimic thyroid storm.
a. The classic features are (i) fever, (ii) tachycardia with atrial fibrillation, (iii) nausea/vomiting, (iv) abdominal pain, (v) delirium/coma, (vi) systolic hypertension with wide pulse pressure, and (vii) high output cardiac failure.36 Pregnant women may be more vulnerable to heart failure with severe thyrotoxicosis due to the already increased cardiac output, diminished vascular resistance, and hypervolemia of pregnancy.37 Hyperglycemia, hypercalcemia, and abnormal liver function tests may also be present. The severity of the clinical picture is not directly related to the circulating level of free T4 or T3. Any patient suspected of thyroid storm requires immediate admission to an intensive care unit (ICU) for invasive hemodynamic monitoring and administration of immediate therapy without waiting for confirmatory laboratory results.
b. Treatment is aimed at (i) blocking the peripheral effect of excess thyroid hormone, (ii) halting thyroid hormone synthesis, (iii) preventing release of preformed hormone, and (iv) reducing peripheral conversion of T4 to T3 (see Table 23.4).33 Adequate adrenergic blockade is essential and requires high doses of β-blockers administered at short intervals because hyperthyroidism increases both the number of β-receptors that need to be blocked and drug metabolism. Propranolol has been the treatment of choice because it decreases T4 to T3 conversion; however, esmolol has also been used successfully.38 PTU should be administered as soon as the diagnosis is made. An initial dose of 300 mg q6h should be administered by mouth or nasogastric tube. The use of inorganic iodine (four to eight drops of Lugol solution every 6 to 8 hours or five drops saturated solution of potassium iodide [SSKI] q6h) or iodinated contrast agents (sodium ipodate 1 mg q8h for 24 hours then 500 mg q12h) are standard therapies in the nonpregnant patient.34 When administered after the first dose of PTU, both are very effective at preventing release of thyroid hormone. Very little information is available on the consequences of large amounts of iodine on the developing fetus; however, the risk to the fetus during uncontrolled, severe maternal thyrotoxicosis likely outweighs the potential risks of fetal iodine exposure. Glucocorticoids may be administered to inhibit peripheral conversion of T4 to T3 and help prevent relative adrenal insufficiency.
CLINICAL PEARLThyroid storm conveys a high risk of maternal and fetal death.
8. Intrapartum and anesthetic considerations
a. The major concerns at delivery are the degree of thyrotoxicosis, presence of maternal goiter, and presence of fetal goiter. Although pregnant women with well-controlled hyperthyroidism generally tolerate labor and delivery without complications, anesthesiologists should be aware of several potential physiologic changes associated with hyperthyroidism that may affect anesthetic management: (i) airway obstruction resulting from an enlarged thyroid gland, (ii) respiratory muscle weakness, (iii) a hyperdynamic cardiovascular system with possible cardiomyopathy, (iv) excessive sympathetic stimulation resulting from pain and anxiety, (v) increased β-adrenergic receptor populations, (vi) electrolyte abnormalities, and (vii) coagulation test abnormalities with increased risk of thrombosis or bleeding depending on severity of thyroid diseases. Ideally, the goal of preoperative preparation is a euthyroid patient; however, minimizing the risk of thyroid storm is most important. In poorly controlled patients, labor and delivery can evoke thyroid storm, and anesthesia providers should be prepared to treat this complication. For those who remain severely thyrotoxic, assessment of maternal cardiac status and monitoring during delivery are important. Preoperative preparation may include the administration of PTU, glucocorticoids, sodium iodide, and a β-blocker.
b. Labor analgesia
Excessive anxiety and inadequate pain control during labor can activate the sympathetic nervous system. Either epidural or CSE analgesia should be initiated early in labor. Continuous infusion epidural techniques or patient-controlled epidural anesthesia (PCEA) can be used to maintain analgesia and minimize the risk for thyroid storm. Labetalol, commonly available and familiar to labor and delivery room staff, can be used for β-blockade.39 In addition to the usual management considerations during labor analgesia administration (e.g., left uterine displacement), hypotension can be treated with a combination of crystalloid administration and vasopressor therapy, but judicious dosing of vasopressors is recommended to avoid possible exaggerated hypertensive responses.
c. Cesarean delivery
(1) Anesthetic choice. Spinal, epidural, or general anesthesia is acceptable, but in most cases, neuraxial anesthesia is preferred to general anesthesia; however, no studies have evaluated the effectiveness or safety of a particular technique in this patient population. In awake patients with adequate neuraxial anesthesia, excessive anxiety and sympathetic nervous system activation can be problematic. Although there has been some concern about the administration of epinephrine-containing local anesthetic solutions in patients with hyperthyroidism, due to fear of exaggerated circulatory responses, most agree that it is safe to administer these local anesthetics to minimize uptake and reduce risk of local anesthetic toxicity. Hypotension should be treated with IV crystalloid and judicious vasopressor therapy. If general anesthesia is necessary, drugs that stimulate the sympathetic nervous system (e.g., ketamine) or cause maternal tachycardia (e.g., atropine, glycopyrrolate, β-mimetics for tocolysis, and pancuronium) should be avoided, if possible. Midazolam, 1 to 2 mg IV, can be used to reduce maternal anxiety.
(2) Ex utero intrapartum treatment procedure. If fetal goiter is identified and not resolved through treatment in utero, the delivery plan must be discussed by a multidisciplinary team that includes obstetricians, neonatologists, and anesthesiologists. An ex utero intrapartum treatment (EXIT) procedure should be considered when there is concern for fetal airway compromise.40
D. Hypothyroidism
1. Pathophysiology
Hypothyroidism is caused by inadequate thyroid hormone production. The prevalence of hypothyroidism during pregnancy is approximately 2% to 3%, although this is likely an underestimate.41 Hypothyroidism may be overt (i.e., elevated TSH and low free T4) or subclinical (i.e., elevated TSH, free T4 in normal reference range).
a. In the developed countries, autoimmune destruction (Hashimoto thyroiditis) is the most common cause.
b. Iodine deficiency remains the leading cause globally with up to 30% of the world’s population at risk.
Other causes include:
c. Ablation with radioactive iodine following treatment for Graves disease or thyroid nodule.
d. Thyroidectomy (partial or near complete for treatment of benign or malignant neoplasm, Graves disease).
e. Medications (e.g., lithium, amiodarone).
f. Transient inflammatory thyroiditis.
g. Central hypothyroidism is rare in prepregnancy and can be due to inadequate stimulation of the thyroid gland because of a defect at the level of the pituitary or hypothalamus.
2. Clinical presentation
The clinical manifestations of mild to moderate hypothyroidism are vague and often insidious in onset, making it often difficult to differentiate the disease from the normal signs and symptoms of pregnancy. These include (a) fatigue, (b) constipation, (c) cold intolerance, (d) weight gain, (e) carpal tunnel syndrome, (f) hair loss, (g) voice changes, (h) reduced memory, (i) muscle cramps, and (j) dry skin. Women who report these over the previous year are more likely to have overt thyroid disease.41 The presence or absence of a pathologically enlarged thyroid gland depends on the etiology of hypothyroidism. Women in areas of endemic iodine deficiency, or those with Hashimoto thyroiditis, are more likely to have a goiter.
3. Treatment
The goal of treatment in pregnant women with overt hypothyroidism is clinical and biochemical euthyroidism at the time of conception and throughout pregnancy. Levothyroxine sodium (thyroxine) is the treatment of choice for routine management of hypothyroidism. The long half-life of thyroxine (7 days) does not permit rapid dosage titration. Most women with hypothyroidism will require an increased dose once pregnant due to increased hepatic-binding protein production secondary to estrogen stimulation, fetal transfer, and placental clearance.
4. Obstetric risk and complications depend on the severity and cause of the hypothyroidism.
a. Fetal risk
Iodine deficiency results in inadequate fetal thyroid hormone production throughout gestation resulting in profound fetal hypothyroidism. This results in: (i) significant neurodevelopmental delay, (ii) deafness, (iii) stunted growth, and (iv) increased risk of neonatal mortality. Although modest but significant changes in psychomotor and IQ testing among children exposed to mild maternal hypothyroidism have been reported, neonatal thyroid function is normal.
b. Obstetric complications
The fetal thyroid gland does not start making thyroid hormone until 14 weeks’ gestation, so maternal hypothyroidism during the first trimester of pregnancy is particularly detrimental.41 Obstetric complications from mild hypothyroidism include (i) increased risk of stillbirth, (ii) preterm delivery, (iii) preeclampsia, (iv) placental abruption, (v) breech presentation, and (vi) low birth weight.42 Women who have been rendered hypothyroid following radioactive iodine or thyroidectomy for their underlying Graves disease may continue to make TSHrAb, which puts the fetus at risk for fetal Graves disease.
5. Antenatal care
a. Women with known thyroid disease should have a serum TSH at their first prenatal visit. A low normal TSH (<2.5 mU per mL) should be the goal during pregnancy. In the first trimester, a slightly suppressed TSH is normal and the dose of Levothyroxine (L)-thyroxine should not be altered. Almost half of these women will require an increase in thyroid replacement during pregnancy. When dose adjustments are made, the TSH should be repeated every 4 to 6 weeks, and then every 8 to 12 weeks, when stable.
b. Severe, undiagnosed hypothyroidism is extremely rare in pregnancy because it tends to lead to infertility. However, there are several case reports of women presenting with myxedematous coma in pregnancy.43 Severe hypothyroidism may be precipitated by (i) infection, (ii) medications including sedatives and opioids, and (iii) cardiovascular events.
c. The clinical presentation of severe hypothyroidism includes (i) alteration in cognitive function, (ii) depression, (iii) hypothermia, (iv) bradycardia, (v) hypotension, (vi) hypercapnia, and (vii) hyponatremia.
d. Therapy should involve (i) electrocardiogram (ECG) monitoring, (ii) thyroxine replacement, (iii) external warming, (iv) judicious fluid replacement, and (v) glucocorticoid therapy.
6. Intrapartum and anesthetic considerations
a. Although prior thyroid surgery or medical therapy should alert providers about possible hypothyroidism, primary hypothyroidism is often undiagnosed. Anesthetic management of the parturient with untreated hypothyroidism focuses on (i) increased myocardial and hemodynamic effects of depressant drugs (e.g., volatile anesthetics), (ii) risk of coronary artery disease, (iii) altered metabolism and inactivation of drugs, (iv) monitoring for the respiratory depression with opioid administration, (v) detection of obstructive sleep apnea, (vi) recognition of skeletal and respiratory muscle dysfunction, (vii) monitoring for altered ventilatory responses to hypoxemia, (viii) obtaining tests for adrenal insufficiency, (ix) detecting electrolyte abnormalities, (x) observing for altered consciousness, and (xi) monitoring for abnormal platelet count and coagulation factors.
b. Anesthesia for labor and delivery
Patients who are not euthyroid are at increased risk for impaired baroreceptor responses as well as decreased intravascular volume. During labor, neuraxial analgesic techniques are preferred to parenteral techniques because of risk of opioid-induced respiratory depression. Labor can induce a stress response, precipitating decreased adrenal cortical function. In these patients, glucocorticoid replacement will be necessary. Although there are no special considerations for administration of neuraxial analgesic techniques in patients who are hypothyroid, hypothyroidism may be associated with qualitative platelet dysfunction. In patients with overt disease, it may be prudent to verify normal coagulation before proceeding with neuraxial techniques, although there are no documented cases of a neuraxial hematoma in patients with thyroid disease. Responses to vasopressor therapy are normal.
c. Anesthesia for cesarean delivery
Although neuraxial anesthesia is the preferred technique for CD, some parturients will require general anesthesia. In such cases, particular attention should be paid to the airway examination if a large goiter is present. In addition, cardiorespiratory depressant drugs should be used cautiously. Rapid sequence induction can be accomplished with minimal doses of induction agent (e.g., thiopental or ketamine). Etomidate may also be considered but may suppress serum cortisol. In these patients, the physiologic responses to hypercarbia and hypoxia are abnormal. Judicious benzodiazepine and opioid administration are required. Volatile anesthetics should also be administered with caution due to their cardiac depressant effects. If muscle relaxation is necessary beyond an intubating dose of succinylcholine, administration of nondepolarizing muscle relaxants should be guided with the use of a nerve stimulator due to abnormal skeletal and respiratory muscle function.
CLINICAL PEARLBoth regional and general anesthesia are safe alternatives for anesthesia of the parturient with adequately controlled thyroid disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








