GENERAL APPROACH TO WOMEN OF CHILDBEARING AGE
The differential diagnosis for women of childbearing potential who present with abdominal or pelvic symptoms or abnormal vaginal bleeding is broad (Table 98-1). The major clinical goals are, first, diagnosis of pregnancy, and then if pregnant, differentiating ectopic pregnancy from threatened abortion. Consider ectopic pregnancy in women of childbearing age who report abdominal or pelvic pain or discomfort, vaginal spotting or a cycle of amenorrhea, or unexplained signs or symptoms of hypovolemia. There are rare case reports of ectopic pregnancy in patients with ovaries but without a uterus. No combination of signs or symptoms is sufficient to exclude ectopic pregnancy. If pregnancy is detected, ectopic pregnancy remains in the differential diagnosis until it can be either confirmed or excluded with conviction.
| All Patients | Pregnant Patients |
|---|---|
| Appendicitis | Normal (intrauterine pregnancy) |
| Inflammatory bowel disease | Threatened abortion |
| Ovarian pathology | Inevitable abortion |
| Cyst | Molar pregnancy |
| Torsion | Heterotopic pregnancy* |
| Pelvic inflammatory disease | Implantation bleeding |
| Endometriosis | Corpus luteum cyst |
| Sexual assault/trauma | |
| Urinary tract infection | |
| Ureteral colic |
PREGNANCY TESTING
The diagnosis of pregnancy is central to the diagnosis of ectopic pregnancy. Pregnancy tests currently in use rely on the detection of the β subunit of human chorionic gonadotropin (β-hCG) in the urine or serum. hCG is a hormone produced by the trophoblast. Intact hCG consists of the α and β subunits. Tests based on detection of the intact molecule or the α subunit can cross-react on immunologic assays with hormones found in the nonpregnant individual and are thus less specific than tests for the β-hCG subunit.
hCG preparations are currently standardized in relation to the Third International Reference Preparation. Other standard preparations are not equivalent. A preparation often referred to in earlier literature is the Second International Standard. The Third International Reference Preparation is roughly equal to 1.7 times the Second International Standard. To avoid confusion when interpreting the literature, pay attention to the standard used. In this chapter, hCG and β-hCG concentrations refer to the Third International Reference Preparation unless otherwise noted.
Very early in either an intrauterine pregnancy (IUP) or an ectopic pregnancy, detectable amounts of β-hCG are released into the serum and filtered into the urine. The concentration of β-hCG is fairly closely correlated in the urine and serum, with urinary concentration also depending on urine specific gravity. Qualitative urine and serum tests for pregnancy usually use the enzyme-linked immunosorbent assay methodology. In the laboratory setting, enzyme-linked immunosorbent assay tests can detect β-hCG at concentrations <1 mIU/mL.
Qualitative tests in clinical use are typically reported as “positive” when the β-hCG concentration is ≥20 mIU/mL in urine and ≥10 mIU/mL in serum. A positive qualitative test therefore implies that β-hCG is present in at least this concentration. At this level of detection, the false-negative rate for detection of pregnancy will not be >1% for urine and 0.5% for serum. In clinical use, the performance of urine qualitative testing is 95% to 100% sensitive and specific compared with serum tests.
Urine tests can be performed rapidly at the bedside, and kits from some manufacturers may be used for either urine or serum. Dilute urine may cause a false-negative urine pregnancy test, particularly early in pregnancy when β-hCG levels are low (<50 mIU/mL). Additionally, when hCG levels are present in large amounts (generally with concentrations of 1,000,000 mIU/mL), a “hook effect phenomenon” can occur, giving false-negative results. This is thought to be related to excess hCG saturating both the fixed, solid-phase antibody and the labeled, soluble antibody of the assay, causing an absence of signal. The resultant false-negative test can be mitigated by diluting the sample.1 Point-of-care hCG whole-blood assay shows promising results, with 95.8% sensitivity (negative predictive value, 97.9%) when compared with standard urine testing.2
When a bedside urine test is negative and ectopic pregnancy is still being considered, perform a quantitative serum test. The sensitivity of quantitative serum testing for the diagnosis of pregnancy is virtually 100% when an assay capable of detecting ≥5 mIU/mL of β-hCG is used.3 Estimated β-hCG levels after conception are listed in Table 98-2.
| Postconception Week | β-hCG Levels (mIU/mL) |
|---|---|
| <1 week | 5–50 |
| 1–2 weeks | 50–500 |
| 2–3 weeks | 100–5000 |
| 3–4 weeks | 500–10,000 |
| 4–5 weeks | 1000–50,000 |
| 5–6 weeks | 10,000–100,000 |
| 6–8 weeks | 15,000–200,000 |
| 8–12 weeks | 10,000–100,000 |
ECTOPIC PREGNANCY
Ectopic pregnancy occurs when a conceptus implants outside of the uterine cavity and is a leading cause of maternal death in the first trimester of pregnancy.5
The current incidence of ectopic pregnancy is difficult to determine but is probably increasing. Reasons include the increased incidence of sexually transmitted tubal infections, unsuccessful tubal sterilizations, assisted reproductive techniques, previous pelvic surgery, and more sensitive and earlier diagnostic techniques. Major risk factors are shown in Table 98-3, but a significant number of ectopic pregnancies occur in women in whom no risk factors are identified.6,7
Pelvic inflammatory disease, history of sexually transmitted infections History of tubal surgery or tubal sterilization Conception with intrauterine device in place Maternal age 35–44 (age-related change in tubal function) Assisted reproduction techniques (cause unknown, as tube is bypassed in implantation) Previous ectopic pregnancy Cigarette smoking (may alter embryo tubal transport) Prior pharmacologically induced abortion |
Fertilization of the oocyte usually occurs in the ampullary segment of the fallopian tube. In normal pregnancy, after fertilization, the zygote passes along the fallopian tube and implants into the endometrium of the uterus. An ectopic pregnancy occurs when the zygote implants in any location other than the uterus—the fallopian tube or extratubal sites (the abdominal cavity, cervix, or ovary). Death results from maternal exsanguination after tubal rupture.
The vast majority of ectopic pregnancies implant in the ampullary portion of the fallopian tube. The underlying cause is most often damage to the tubal mucosa from previous infection, preventing transport of the ovum to the uterus. Other causes include tubal surgery, defects in the ovum resulting in premature implantation, and elevated estradiol or progesterone levels, which inhibit tubal migration.
Tubal implantation results in the penetration of the ovum into the muscular wall of the tube, and maternal blood seeps into tubal tissue. Intermittent distention of the fallopian tube with blood can occur, with leakage of blood from the fimbriated end of the fallopian tube into the peritoneal cavity. The aborting ectopic pregnancy and associated hematoma can be completely or partially extruded out of the end of the fallopian tube or through a rupture site in the tubal wall.
Abdominal ectopic pregnancies (~1% of ectopic pregnancies) most commonly derive from early rupture or abortion of a tubal pregnancy, with subsequent reimplantation in the peritoneal cavity.
Cervical ectopic pregnancies occur in <1% of ectopic pregnancies, with predisposing factors similar to those associated with ectopic pregnancies (previous dilatation and curettage, previous cesarean delivery, in vitro fertilization, adhesions or fibrosis of the endometrium, prior instrumentation, infertility, previous ectopic pregnancy). Patients develop profuse vaginal bleeding. Bimanual exam reveals a soft, large cervix when compared to the uterus or an hourglass-shaped uterus, and diagnosis is confirmed with US.8,9
Cesarean scar pregnancy is rare but can cause massive maternal hemorrhage. Diagnosis is difficult and is based on US demonstrating an empty uterine sac and cervical canal and a gestational sac in the uterine isthmus.
Determine the timing and characteristics of the last few periods. The menstrual history is often, but not always, abnormal. The classic sign of amenorrhea from 4 to 12 weeks after the last normal period is reported in 70% of ectopic pregnancy cases. No missed menses are reported in 15% of ectopic pregnancy cases. Although vaginal bleeding is often scant, heavy bleeding does not exclude an ectopic pregnancy.
Ask about previous pregnancies, pregnancy problems, and miscarriages. Typical early pregnancy symptoms may occur and may not differ from symptoms of previous normal IUPs. Discuss previous medical and surgical history, and ask about substance abuse and smoking. Ask about sexual activity and contraception. Identify risk factors for ectopic pregnancy or spontaneous abortion. Determine current medications, including over-the-counter drugs.
Pregnancy in a patient with prior tubal surgery for sterilization is assumed to be an ectopic pregnancy until proven otherwise. Patients are at particularly high risk if they have undergone laparoscopic partial salpingectomy or electrodestruction tubal ligation at a young age (age <28 years), especially 5 to 15 years after the procedure.10
In a woman of childbearing age, hysterectomy with oophorectomy excludes ectopic pregnancy. In the situation of hysterectomy without oophorectomy, ectopic pregnancy is exceedingly rare. A literature review identified only 27 such case reports since 1918, after both vaginal and abdominal hysterectomies. The theory is that a fistulous tract after hysterectomy enables embryo implantation in the tube or adnexae.11
Abdominal pain or discomfort is the most common symptom of ectopic pregnancy and is reported in 90% of ectopic pregnancies.12 Pain is due to tubal distention or rupture. The classic pain of rupture is lateralized, sudden, sharp, and severe. Shoulder pain due to diaphragmatic irritation from a ruptured ectopic pregnancy can also occur. Any lateral or bilateral abdominal discomfort or tenderness in a woman of childbearing age requires consideration of ectopic pregnancy. Lack of pain in a woman with vaginal spotting or bleeding does not exclude ectopic pregnancy.
Obtain vital signs and focus on the abdominal and pelvic examination. The physical examination in ectopic pregnancy is highly variable, and ectopic pregnancy is difficult to diagnose or exclude based on physical examination. In cases of ruptured ectopic pregnancy, patients may present in shock, with no findings on pelvic examination, or with peritoneal signs and an adnexal mass and tenderness. Cervical motion tenderness can be elicited on pelvic exam in some cases. Relative bradycardia may occur as a consequence of vagal stimulation. There is poor correlation between the volume of hemoperitoneum and vital signs in ruptured ectopic pregnancy.13 In cases of rupture without hemodynamically significant bleeding, a more benign abdominal exam may be present without significant alteration of vital signs. Fever is rare. In the more common situation of an unruptured ectopic pregnancy, the vital signs are likely to be normal.
If an adnexal mass or fullness with tenderness is detected, it may be due to ectopic pregnancy or to a corpus luteum cyst, a 3- to 11-cm, thin-walled, unilocular cyst seen after ovulation that can cause pain and tenderness on exam as well as menstrual irregularities, mimicking an ectopic pregnancy. Cervical motion tenderness may be seen, and blood is often present in the vaginal vault; however, the pelvic exam may be completely normal. The cervix may have a blue coloration, as in a normal pregnancy. Uterine size for estimated gestational age is most often normal. Vaginal examinations in stable women presenting with first-trimester bleeding may add little to the clinical diagnosis; some providers are moving away from routine use of vaginal examinations in initial patient assessment as long as a transvaginal US is obtained.14
The definitive diagnosis of ectopic pregnancy is made by US, by direct visualization by laparoscopy, or at surgery. No single or combination of laboratory tests has a sufficient negative or positive predictive value to completely exclude ectopic pregnancy or to definitively establish the diagnosis.
Differences in the dynamics of β-hCG production in normal and pathologic pregnancy are useful in the diagnosis of ectopic pregnancy. Early in normal pregnancy, β-hCG levels rise rapidly until 9 to 10 weeks of pregnancy and then plateau. Expected postconception ranges of β-hCG are listed in Table 98-2. β-hCG levels decline in nonviable pregnancies and in successfully treated ectopic pregnancy. Absolute levels of β-hCG tend to be lower in pathologic pregnancies than in IUPs, but there is much overlap. Due to the variability in absolute levels and the overlap between normal and pathologic pregnancies, no single β-hCG level can reliably distinguish between a normal and a pathologic pregnancy. Doubling time refers to the time needed for β-hCG concentration in the serum to double. Absolute levels of β-hCG are lower and doubling times longer in ectopic pregnancy and other abnormal pregnancies. This and many other observations have led to the widely used rule of thumb stating that the serum concentration of β-hCG approximately doubles every 2 days early in a normal pregnancy and that longer doubling times indicate pathologic pregnancy. Varying degrees of sensitivity (36% to 75%) and specificity (63% to 93%) are obtained using different criteria for evaluating the rate of increase of β-hCG levels for the diagnosis of ectopic pregnancy. The minimum hCG rise in normal pregnancy may be as low as 53% in 48 hours,15 and the median rise in β-hCG level was 53% in 1 day and 124% in 2 days. Conversely, in spontaneous abortion, hCG is expected to fall by 21% to 35% in 2 days.16 Thus, hCG levels that fail to increase by 53% or more in 2 days are suggestive but not diagnostic of ectopic pregnancy or an abnormal IUP. However, an increase of >53% does not rule out ectopic pregnancy.
In stable patients, serial measurements of β-hCG are used to either heighten or lower the suspicion for ectopic pregnancy, but are not diagnostic. Repeat serum β-hCG measurement made at least 2 days after the initial presentation is useful in characterizing the risk of ectopic pregnancy and the probability of a viable IUP.17 Although rates of decline vary depending on the initial hCG value, a decrease of less than 21% at 2 days or 60% at 7 days suggests retained trophoblasts or an ectopic pregnancy; additional testing should be performed.15 Table 98-4 describes the American College of Emergency Physicians’ clinical policy regarding women with early pregnancies.17
| Clinical Issue | Recommendation | Level of Recommendation* |
|---|---|---|
| Use of transvaginal US to detect IUP, ectopic pregnancies when serum β-hCG <1000 mIU/mL | Perform or obtain a transvaginal US for symptomatic pregnant patients with β-hCG below the discriminatory threshold. | Level C |
| Use of β-hCG for predicting ectopic pregnancy in women with indeterminate transvaginal US | β-hCG values do not exclude the diagnosis of an ectopic pregnancy in patients with indeterminate transvaginal US. | Level B |
| Implications for ED management of women receiving methotrexate for confirmed or suspected ectopic pregnancy | Consider ruptured ectopic pregnancy for persistent abdominal pain or vaginal bleeding. | Level B |
| Anti-Rh0 (D) immunoglobulin for ectopic pregnancy | 50 micrograms of RhoGAM for all Rh-negative women with threatened loss or loss of established first-trimester pregnancy (full dose of 300 micrograms is not needed when the patient is at <12 weeks of gestation due to the small volume of red cells in the fetoplacental circulation). | Level B |
Progesterone is a steroid hormone secreted by the ovaries, adrenal glands, and placenta during pregnancy. During the first 8 to 10 weeks of pregnancy, ovarian production of progesterone predominates, and serum levels remain relatively constant. After the tenth week of pregnancy, placental production increases and serum levels rise. Absolute levels of progesterone are lower in pathologic pregnancies and fall when a pregnancy fails. This observation has led multiple authors to propose various progesterone levels as a diagnostic aid in differentiating an early normal from a pathologic pregnancy. Most pathologic pregnancies have progesterone levels of ≤10 nanograms/mL. With progesterone ≤5 nanograms/mL, nearly 100% of pregnancies will be pathologic; there are no normal pregnancies reported with progesterone ≤2.5 nanograms/mL. Progesterone levels >25 nanograms/mL have 97% sensitivity for viable IUP. An empty uterus or nonspecific fluid collection on US associated with progesterone ≤5.0 nanograms/mL is highly predictive of abnormal IUP or ectopic pregnancy.18
There is considerable overlap between progesterone levels in normal and pathologic pregnancy. Thus, very low values for serum progesterone should increase the clinical suspicion for ectopic pregnancy or abnormal IUP, but as with β-hCG levels, no value is diagnostic or can completely exclude or definitively diagnose ectopic pregnancy. Progesterone levels may not be routinely available on an urgent basis, and as noted, many patients have intermediate values, thus limiting the usefulness of the test. Consequently, the role of serum progesterone assays is currently unclear.
Numerous other serum markers for the diagnosis of ectopic pregnancy have been investigated. These include secretory endometrial protein, estradiol, the pregnancy-associated proteins A to D, and others, as well as routine laboratory tests such as amylase, creatine kinase, erythrocyte sedimentation rate, and others. None has been accepted as equal or superior to β-hCG measurements at this time.
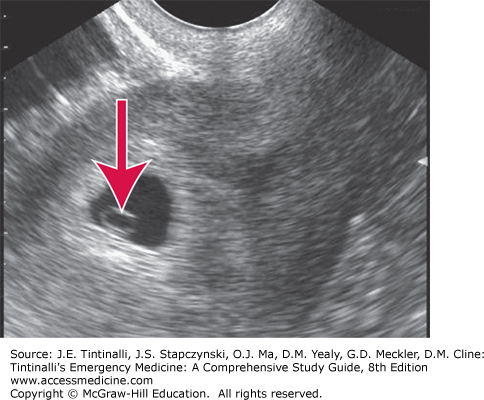
The primary goal of US in early pregnancy is determination of a viable IUP and exclusion of ectopic pregnancy (Figure 98-1).
US findings may also be useful in planning therapy when an ectopic pregnancy is discovered. Additionally, US provides information regarding fetal age and viability when an IUP is present.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree