Echocardiography in the Intensive Care Unit
Achikam Oren-Grinberg
Adam B. Lerner
Daniel Talmor
Echocardiography was introduced to the operating suite in the 1970s, with epicardial echocardiography as its initial application. Transesophageal echocardiography (TEE) during surgery was first described in 1980 but did not become commonplace until the mid-1980s. Since then, TEE has evolved to become a widely used and versatile modality for diagnosis and monitoring of critically ill patients. As such, its use has expanded into the perioperative period and the intensive care unit (ICU). Echocardiography provides both anatomic and functional information about the heart; systolic and diastolic function, cavity size, and valvular function [1].
Ease of utilization, availability of diagnostic information within 10 to 15 minutes from the start of examination, high quality imaging in most patients, and low complication rates have all led to the pervasive use of echocardiography in the perioperative environment and increasing use in the ICU [2, 3, 4, 5, 6, 7 and 8]. However, patient safety and optimal outcome depend heavily on a thorough understanding of both the strengths and limitations of the available technologies and their applications.
Indications and Guidelines
The role of TEE has evolved and expanded, leading to the need for standardization of indications and practice. A task force created in 1993 conducted a literature review of 558 studies in search of evidence for the effectiveness of TEE in the perioperative setting. Three years later, the American Society of Anesthesiologists (ASA) and the Society of Cardiovascular Anesthesiologists (SCA) published guidelines regarding the indications for TEE [9]. Three categories of evidence-based clinical indications were identified. For indications grouped into category I, TEE was judged to be frequently useful in improving clinical outcomes. To date there is only a single category I indication for TEE in the ICU. That indication is for “unstable patients with unexplained hemodynamic disturbance, suspected valve disease, or thromboembolic problems (if other tests or monitoring techniques have not confirmed the diagnosis or patients are too unstable to undergo other tests)”[9]. This indication, however, encompasses a significant proportion of ICU patients.
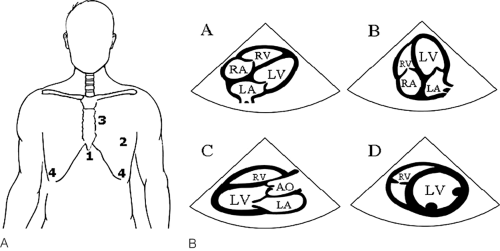
As TEE utilization in the perioperative setting became more widespread, the need for consistency in imaging acquisition became increasingly evident. In 1999, the joint task force of the American Society of Echocardiography (ASE) and SCA published guidelines defining a comprehensive cardiac exam using TEE [10]. Specifically, these guidelines defined a set of cross-sectional views and nomenclature that constitute a comprehensive intraoperative TEE examination. It is important to note that occasional deviation from the guidelines is needed in order to acquire optimum imaging. The expectation was that “the guidelines may enhance quality improvement by providing means to assess the technical quality and completeness of individual studies” and that “more consistent acquisition and description of intraoperative echocardiographic data will facilitate communication between centers and provide a basis for multicenter investigations” [10].
Basic Terminology of Echocardiography Techniques
A sonographer must use different echocardiographic imaging techniques and hemodynamic modalities in order to achieve a diagnosis or management plan. The following is a list of the basic techniques used during an echocardiographic study.
Two-Dimensional Echocardiography
Two-dimensional (2D) echocardiography is the backbone of the echocardiographic exam [11]. Using 2D, a complete visualization of the beating heart is achieved by displaying anatomic structures in real time tomographic images. By aiming the ultrasound probe at the heart, exactly oriented anatomic “slices” are obtained. Information acquired includes cardiac chamber sizes, global and regional systolic function, and valvular anatomy.
M-Mode Echocardiography
M-mode or motion-mode images are a continuous one-dimensional graphic display that can be derived by selecting any of the individual sector lines from which a 2D image is constructed [11]. It is useful for quantification of myocardial wall and chambers sizes, which in turn can be used to estimate left ventricle (LV) mass and chamber volumes, respectively. In addition, since it has high temporal resolution, M-mode is helpful in assessing the motion of rapidly moving cardiac structures such as cardiac valves.
Doppler Echocardiography
Doppler echocardiography is used to supplement 2D and M-mode echocardiography. It can provide functional information regarding intracardiac hemodynamics; systolic and dia-stolic flows, blood velocities and volumes, severity of valvular lesions, location and severity of intracardiac shunts, and assessment of diastolic function. The four types of Doppler modalities used include continuous-wave, pulsed-wave, color flow mapping, and tissue Doppler [11]. Continuous-wave Doppler is used for measuring high pressure gradient/high velocity flows such as seen in aortic stenosis. When using continuous-wave Doppler, the ultrasound probe continuously transmits and receives sound waves. This increases the maximum limit of blood velocity that can be evaluated before exceeding the Nyquist limit. The Nyquist limit represents the maximum flow velocity that can be evaluated by Doppler and is dependent on both equipment and imaging variables. Continuous-wave Doppler can evaluate higher flows but does so at the expense of spatial specificity. Pulsed-wave Doppler is used for measuring lower pressure gradient/lower velocity flows such as in mitral stenosis. In this mode, the ultrasound probe sends out a pulse of sound and then waits to receive reflected waves. This lowers the Nyquist limit and the maximum velocities that can be interrogated but allows for precise spatial resolution. Color flow mapping is useful for screening valves for stenosis or regurgitation, quantifying the degree of valvular regurgitation, imaging systolic and diastolic flow, and detection of intracardiac shunts. Doppler tissue imaging has been introduced as a new method of quantifying segmental and global left ventricular function. It records systolic and diastolic velocities within the myocardium and at the corners of the mitral annulus and is useful for studying diastolic function and contractile asynchrony of the left ventricle.
Contrast Echocardiography
Contrast echocardiography is used to enhance the diagnostic quality of the echocardiogram [12]. It may be used to improve assessment of global function and regional wall motion abnormalities by 2D echocardiography. Although approved only for LV opacification, recent clinical studies suggest a potential use in assessing myocardial perfusion [13, 14].
Echocardiography Compared with Traditional Monitoring in The Intensive Care Unit
For almost four decades, the pulmonary artery catheter (PAC) has been used as a mainstay of patient monitoring in the ICU setting. It provides direct information on pressure variables such as pulmonary artery pressure, pulmonary artery wedge pressure (PAWP), and central venous pressure. It can also provide flow related data such as cardiac output (CO) and mixed venous oxygen saturation. From these data, other hemodynamic variables, such as systemic vascular resistance, pulmonary vascular resistance, and stroke volume can be calculated. Despite its extensive use (more than 1.5 million PACs inserted annually in North America), the clinical value of data obtained from pulmonary artery catheters remains unproven [15]. One of the major drawbacks of the pressure measurements obtained from PACs is the inability to definitively relate these measurements to intravascular and cardiac chamber volumes. This is due mainly to inter- and intrapatient variability of myocardial compliance. Early studies of the use of PAC in surgical patients yielded inconsistent results. While some studies demonstrated decreased mortality [16, 17 and 18], others showed no effect [19, 20]. In contrast, other studies demonstrated increased morbidity and mortality [21, 22]. Two systematic reviews that included a mixed population of surgical and medical patients and patients with myocardial infarction demonstrated no overall benefit and increased morbidity and mortality from the use of PAC [23, 24]. Finally, a recent randomized, prospective study of nearly 2,000 patients found no benefit to therapy directed by PAC in high-risk surgical patients requiring intensive care [15].
Hemodynamic optimization is a complex task requiring, among other things, monitoring of arterial and venous pressures, urine output, acid-base balance, and oxygen content/delivery. These parameters, however, reflect the overall circulatory state and not the basic physiologic determinants of CO, which include preload, afterload, and contractility. In many conditions, such as with pericardial and pleural effusions, pulmonary embolism, and valvular pathologies, current invasive monitors provide only minimal and indirect information to facilitate satisfactory management. In addition, the current monitoring modalities provide little to no information for assessment of ventricular compliance and relaxation. At present, echocardiography is the only method that can provide real-time bedside imaging of the heart [25, 26]. It allows for the assessment of LV systolic and diastolic function, measurement of CO, and reliable assessment of other hemodynamic variables such as pulmonary artery pressure and PAWP [27]. Data obtained from TEE examination frequently differ from PAC assessments of LV preload and systolic function and, when used, can lead to a change in therapy in 40% to 60% of patients [7, 28].
Transesophageal Versus Transthoracic Echocardiography
Although transthoracic echocardiography (TTE) is a less invasive way to image cardiac structures, suboptimal acoustic windows lead to low-quality images in many critically ill patients. These suboptimal acoustic windows are due to obesity, pulmonary disease, the presence of chest tubes, drains and wound dressings, and limitations on patient positioning. Using TTE in the ICU can be challenging; one report found the echocardiographic examination to be inadequate in approximately 50% of patients on mechanical ventilation and 60% of all ICU patients [8]. The relatively low percentage of adequate imaging improves when TTE is used as a monitoring tool, which does not require the same quality of images, and not as a diagnostic tool. In a report of more than 200 ICU patients, TTE used as a monitoring tool provided 2D images of acceptable quality in 97% of patients [25].
In contrast to transthoracic echocardiography, TEE is more invasive but consistently provides images of better quality. In up to 40% of patients, TEE may provide additional unexpected diagnoses that are missed by TTE [4, 29]. Recent advances in ultrasound imaging, which include harmonic imaging, digital acquisition, and contrast endocardial enhancement, have improved the diagnostic yield of TEE [30, 31]. TEE may also be
used as a continuous monitor of heart function and, when indicated, the probe can be left in the esophagus or stomach for several hours.
used as a continuous monitor of heart function and, when indicated, the probe can be left in the esophagus or stomach for several hours.
Contraindications to Performing Transesophageal Echocardiography
Although TEE is safe [32, 33], there are several contraindications to probe insertion. These include significant esophageal or gastric pathology, mass or tumors, strictures, diverticulum, Mallory-Weiss tears, recent esophageal or gastric surgery, upper GI bleeding, and dysphagia or odynophagia not previously evaluated. Esophageal varices are not an absolute contraindication, and a risk/benefit analysis of each case must be done before performing TEE in any individual patient [34]. Practitioners must be aware of the potential for severe bleeding, in particular when a coagulation abnormality exists. Cervical spinal injury is another relative contraindication requiring careful risk/benefit analysis.
Complications and Safety of Transesophageal Echocardiography
TEE is considered a moderately invasive procedure and complications are rare. In one study of ICU patients, complication rates reached 1.6% and included hypotension following sedation for probe insertion, oropharyngeal bleeding in a coagulopathic patient, and aspiration during tracheal intubation performed prior to TEE [33]. Another study in 2,508 ICU patients reported a complication rate of 2.6%. In this study, as would be expected, there was no examination related mortality. Complications included transient hypotension or hypertension, circulatory deterioration, hypoxemia, arrhythmias, vomiting, coughing, superficial mucous membrane lesions, displacement of a tracheostomy tube, and accidental removal of a duodenal feeding tube [32]. A large European multicenter study of 10,419 exams reported a complication rate of 2.5% with one (0.01%) case of fatal hematemesis due to a malignant tumor [2]. In addition, in 0.88% of the reported cases, the TEE exam had to be prematurely terminated due either to patient intolerance or because of cardiac, pulmonary, or bleeding events [2].
Common Indications for Transesophageal Echocardiography in the Intensive Care Unit
As mentioned, the only current category I indication for the performance of TEE in the ICU setting is in “unstable patients with unexplained hemodynamic disturbances, suspected valve disease, or thromboembolic problems (if other tests or monitoring techniques have not confirmed the diagnosis or patients are too unstable to undergo other tests)” [9]. In practice, however, clinicians use echocardiography in the ICU for many other indications. These are summarized in Table 31-1.
TABLE 31-1. Common Indications for Performing Transthoracic Echocardiography in the Intensive Care Unit | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Echocardiography as a Hemodynamic Monitor in the Intensive Care Unit
Managing the hemodynamically unstable patient in the ICU remains an often challenging and time-consuming exercise. Echocardiography can be used effectively as a monitoring tool for management of these complex ICU patients. The FATE protocol is a relatively easy to use and efficient protocol for monitoring patients in the ICU [25]. The FATE examination is a rapid echocardiographic assessment performed to screen for significant pathology and obtain information about the volume and contractility of the heart. The steps of the protocol include [25]:
Excluding obvious pathology
Assessing wall thickness and chamber dimensions
Assessing contractility
Visualizing the pleura on both sides
Relating the information to the clinical context
The exam can be performed by physicians with only limited training in echocardiography. It requires imaging the heart and pleura in the most favorable sequence from one or more tomographic planes [25]. Different Doppler modalities can be used as needed to calculate CO, assess valvular pathologies, and to measure a variety of hemodynamic variables. Although it is possible to terminate the protocol once the clinical question has been answered, it is recommended that all imaging positions be obtained because of the possibility of further disorders that would otherwise be missed (Fig. 31-1).
Although the FATE protocol was described for TTE, its principles are readily applied to TEE as well. This systematic approach allows for a rapid assessment of myocardial load conditions, dimensions, and contractility and promotes the ability to properly diagnose and intervene expeditiously. The protocol has been shown to be a practical and useful hemodynamic monitoring tool in ICU patients. A study of the FATE protocol demonstrated that it added new information in 37.3% of patients and contributed decisive information in 24.5% of the patients. Only in 2.6% of the exams performed was the information too limited to aid in patient management [25]. These findings and other reports support the benefit of TEE exam when performed by a noncardiologist in the ICU [28,35, 36 and 37].
Echocardiographic Evaluation of Hemodynamic Instability
Hemodynamic instability is an extremely common event in every ICU. Determining the cause of such can sometimes be more challenging than one would expect. Echocardiography can be used successfully in the diagnosis, monitoring, and management
of the unstable patient in the ICU. Using echocardiography to determine the etiology of hemodynamic instability requires assessment of cardiac function, volume status, valvular function, and extra-cardiac processes.
of the unstable patient in the ICU. Using echocardiography to determine the etiology of hemodynamic instability requires assessment of cardiac function, volume status, valvular function, and extra-cardiac processes.
Assessment of Cardiac Function
Systolic dysfunction of either ventricular chamber must be considered in every unstable patient. The etiology of dysfunction may oftentimes be discerned from the echocardiographic evaluation allowing for appropriate therapy to be initiated.
Assessment of Left Ventricular Systolic Function
Utilization of several echocardiographic assessment modalities is necessary for evaluation of left ventricular systolic function. These modalities include quantitative as well as qualitative assessments.
Quantitative Assessment of Left Ventricular Systolic Function
Volumetric Method Utilizing Geometric Models
Quantitative assessment of left ventricular systolic function relies on volume assessment using 2D tomographic images. To determine the volume at end diastole (LVEDV) and end systole (LVESV), the endocardial borders in one or more tomographic planes are traced at end diastole and end systole. Several geometric assumptions and formulas have been developed (e.g., truncated ellipse, “bullet” formula, cylinder, and cone) to determine the LVEDV and LVESV based on these 2D images. Once LVEDV and LVESV have been determined, the stoke volume, and thus cardiac output can be calculated:
SV = LVEDV – LVESV
CO = SV × HR
In addition, ejection fraction can be calculated from these volumes using the formula:
EF = SV/LVEDV × 100%
The advantage of the geometric assumption techniques is that they require only limited visualization for calculation of ventricular volumes. However, these formulas work only in a symmetrically contracting ventricle; the presence of regional wall motion abnormalities decreases their accuracy. In addition, foreshortening of the LV cavity is a common source of underestimation of LV end diastolic and end systolic volumes and can similarly impact the accuracy of systolic function assessment with these formulas [1, 38]. Finally, since the models depend on accurate endocardial border definition, their use requires adequate visualization. Incomplete endocardial definition is described in 10% to 20% of routine echocardiographic studies [39] and may reach 25% in ICU patients [40]. This challenge is even greater in patients requiring mechanical ventilation in which imaging can be particularly challenging. These challenges have limited the utilization of the geometric models and formulas for assessment of LV systolic function.
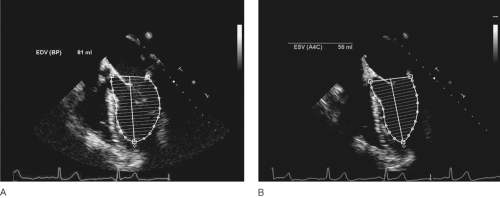
Discs Method (Simpson’s Rule)
Another method for volumetric assessment of LV systolic function is the discs method, which may be more accurate than the other volumetric methods described above, particularly in the presence of distorted LV geometry [41]. In this method the ventricle is divided into a series of discs of equal height and each disc volume is calculated as follows: Disc volume = disc height × disc area. The ventricular volume can be calculated from the sum of the volumes. This technique requires true apical images, which in clinical practice may be difficult to achieve. Foreshortening of the ventricular apex will result in inaccurate assessment of the left ventricular ejection fraction and cardiac output (Fig. 31-2).
Qualitative Assessment of Left Ventricular Systolic Function
2D Evaluation of Ventricular Systolic Function
Using 2D imaging, two of the most important questions regarding hemodynamic stability can be rapidly answered: Are the ventricles contracting well and are they adequately filled? Using 2D, an experienced observer can qualitatively evaluate systolic function. This should be assessed from multiple tomographic planes, and attention must be made to obtaining adequate endocardial definition. Normal ventricular contraction consists of simultaneous myocardial thickening and endocardial excursion toward the center of the ventricle. It is important to look for this myocardial thickening; infarcted myocardium may be pulled inward by surrounding, normal myocardium. There is some regional heterogeneity of normal wall motion with the proximal lateral and inferolateral (or posterior) walls contracting somewhat later than the septum and inferior wall [42]. For qualitative assessment of overall systolic
function, the echocardiographer integrates the degree of wall thickening and endocardial motion in all tomographic views and reaches a conclusion about overall LV systolic function and ejection fraction (EF). Although different institutions use different standards, severe LV systolic dysfunction is usually defined as an EF less than 30%, moderate dysfunction 30% to 45%, mild depression 45% to 55%, and normal greater than 55%. This method of EF estimation is of great clinical utility and can be performed with good correlation to quantitative measurements. There are, however, a few potential pitfalls to 2D assessment of EF that must be considered:
function, the echocardiographer integrates the degree of wall thickening and endocardial motion in all tomographic views and reaches a conclusion about overall LV systolic function and ejection fraction (EF). Although different institutions use different standards, severe LV systolic dysfunction is usually defined as an EF less than 30%, moderate dysfunction 30% to 45%, mild depression 45% to 55%, and normal greater than 55%. This method of EF estimation is of great clinical utility and can be performed with good correlation to quantitative measurements. There are, however, a few potential pitfalls to 2D assessment of EF that must be considered:
Accurate assessment requires satisfactory endocardial border definition. Qualitative EF estimation becomes inaccurate when the endocardium is inadequately defined.
Accurate estimation of EF depends on the experience of the echocardiographer.
In asynchronous contraction (paced-rhythm, conduction defects, etc.), assessment of EF is more difficult.
Despite its limitations, 2D qualitative assessment is the most widely used technique for assessment of LV systolic function due to its ease of application in the clinical setting. In the operating room, after completing the TEE exam, most physicians monitor LV systolic function continuously with 2D imaging using the transgastric (TG) midpapillary short-axis view. This allows for quick assessment of regional wall motion abnormalities in all coronary arterial circulatory beds as well as rudimentary evaluation of volume status [43]. However, it is important to remember that this view alone is never satisfactory for assessing overall systolic function.
Regional Left Ventricular Function
Most commonly, abnormal regional wall motion is the result of coronary artery disease and resultant ischemia/infarction. Abnormal wall motion is a continuum of conditions consisting of hypokinesis, akinesis, and dyskinesis. With dyskinesis, the affected wall segment moves away from the center of the ventricle during systole. In order to standardize echocardiographic evaluations of wall motion, a 17-segment model of the LV has been defined [42]. These 17 segments are evaluated separately for the presence and degree of regional wall motion abnormality. When the etiology of the wall motion abnormality is coronary artery disease, the location of the coronary lesion can usually be predicted from the location of the regional wall motion abnormality.
Contrast Echocardiography
Recent innovations have been made to overcome some of the technical obstacles related to endocardial border detection and image quality. Intravenous echocardiographic contrast agents that opacify the left side of the heart can markedly improve visualization of the LV cavity and enhance endocardial definition. These agents can aid assessment of regional and global LV function [44, 45, 46 and 47]. They also have the potential to “salvage” nondiagnostic TTEs in ICU patients. One study demonstrated a “salvage” rate of 51% [48], and another 77% of nondiagnostic TTEs [49]. Optison ™, a sonicated Perfluoron propane-filled albumin microsphere contrast, and other similar agents have been used safely to improve endocardial border visualization [45, 50]. In addition to improving visualization and assessment of LV function, assessment of myocardial perfusion defects with intravenous contrast has been reported with a variety of imaging techniques and modalities [51, 52 and 53].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree