and Richard A. Jaffe2
(1)
David Geffen School of Medicine at UCLA, Los Angeles, California, USA
(2)
Stanford University School of Medicine, Stanford, California, USA
Keywords
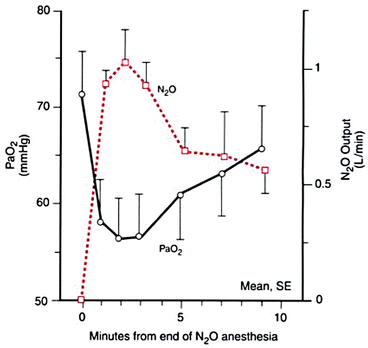
HypoxiaNitrogenNitrous oxideDiffusion hypoxia is a poorly understood event in clinical anesthesia. It occurs in only one circumstance, which is only when a patient simultaneously breathes two relatively insoluble gases, both in high concentration [1]. For example, supposing a patient is breathing nitrous oxide 60 % as part of a standard anesthetic technique, and at the end of the anesthetic is immediately allowed to breathe room air, which contains 79 % nitrogen. As the patient inhales, the nitrogen enters the lungs, but because of its insolubility does not enter the pulmonary blood stream in any appreciable amount. Simultaneously nitrous oxide, which is also insoluble but less so than nitrogen, leaves the pulmonary blood, enters the alveoli and is exhaled. If nitrous oxide and nitrogen had the same solubility coefficient, there would be an even exchange of molecules across alveoli and diffusion hypoxia would not occur. However, because nitrogen is less soluble than nitrous oxide, the molecular exchange of gases in the lung is not even. Nitrogen tends to stay in the alveoli and nitrous oxide continues to diffuse from blood stream into alveoli. As a result, the two insoluble gases concentrate in the lung thereby decreasing the concentration of oxygen resulting in transient diffusion hypoxia (Fig. 20.1) [1]. The same event could occur if one of the gases is a high concentration of helium. To avoid this problem, the anesthesia provider should always administer a high concentration of oxygen for a few minutes before transitioning from nitrous oxide (or helium) to room air.