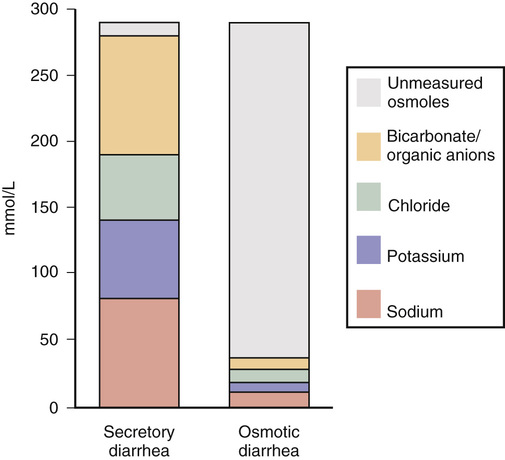
Michelle Freshman Diarrhea is generally appreciated as an increase in stool frequency of more than three stools per day, typically appearing loose or liquid, consistent with a stool weight greater than 200 g daily. Diarrhea can range from a mild, self-limited episode to a severe, life-threatening illness. Acute diarrhea, lasting less than 2 weeks, whether it is infectious or noninfectious, usually improves without intervention. Persistent diarrhea over 2 to 4 weeks might be associated with a protozoal or other endemic infection and is more commonly described by pediatric gastroenterologists.1 When diarrhea continues for a month without improvement, it is considered chronic. Hyperdefecation, an increase in stool frequency without a concomitant change in stool consistency, and fecal incontinence, involuntary loss of stool, are distinct. Among industrialized country inhabitants with adequate sanitation, chronic diarrhea may account for 3% to 5% of cases annually,1,2 whereas an individual might experience acute diarrhea once every 18 months.3 Community-dwelling and hospitalized elderly patients are at increased risk. Approximately 10% of acute diarrhea is noninfectious, caused by trauma, medications, toxins, transient ischemia, diverticulitis, or flares of irritable bowel syndrome (IBS) or inflammatory bowel disease (IBD). Chronic diarrhea is usually noninfectious and related to a host of malabsorptive, autoimmune, endocrine, malignant, and surgical conditions. Chronic diarrhea can be intermittent or continuous and can be manifested with extraintestinal processes or complications. Approximately 10 L of fluid daily enters the jejunum, yet only a fraction leaves the body within stool. In 95% of individuals, 200 mL of fluid or less is excreted—more with higher dietary fiber.3 The colon is able to recover up to four times its usual volume but is dependent on a tempered flow rate, allowing enough time for the maximum reabsorption of 800 mL to occur. Between the small and large intestines, this volume accounts for 99% of the excess water reabsorption; a decrease of as little as 1% may result in significant diarrhea.3 A key mechanism in balancing colonic electrolyte and water flow is sodium (and chloride) regulation by cholinergic, adrenergic, and serotonergic mediators, among others. Multiple mechanisms create the conditions for diarrhea. Although some focus on three categories—watery (osmotic and secretory diarrhea), inflammatory, and fatty diarrhea—noting many overlapping presentations, others separately account for secretory, osmotic, fatty, motility, inflammatory (including noninvasive infections), and functional disorders. Secretory diarrhea is the most common type. The circumstances typically involve chemical, mechanical, or functional disruptions that produce an excess of electrolyte, nutrient, and water content in the colon. Secretory diarrhea is defined as an imbalance in fluid and electrolytes that cannot be adequately reabsorbed when water enters the bowel after a high solute or high anion load. Caused by proximal hypersecretion of the gut and a breakdown in the normal reabsorption of electrolytes and water from the colon, the result can be large-volume isotonic or hypertonic fluid in the colon, which affects patients even during fasting. For example, loss of intrinsic factor from surgery or damaged ileum will result in unconjugated bile salts that spill into the colon, pulling additional water into the lumen. Nonosmotic medications, endocrine disorders, and cancers are examples. By contrast, osmotic diarrhea includes malabsorptive disorders and results from solute-rich molecules (e.g., laxatives with magnesium, phosphate, or sulfate-containing ingredients)3 leaving the vascular space and entering the colon, which in turn draws more water and salt into the intestinal lumen or may prevent water from entering the vascular space (Fig. 132-1). This can result in excretion up to 1 L/day.2 Diarrhea starts postprandially and typically ends with fasting or discontinuation of the identified ingredient. Short-chain fatty acids produced as a result of bacterial metabolism of the undigested small carbohydrates result in the retention of fluid distally as well as a lower fecal pH. Steatorrhea, another secretory diarrhea known as fatty diarrhea, is usually associated with malabsorption or maldigestion involving mucosal changes, ileal disease or surgery, bile acid deficiency, or pancreatic exocrine insufficiency. Type 1 bile acid diarrhea is associated with ileal disease. Cases involving 100 cm of ileum or less produce diarrhea typically; more than 100 cm of resected or diseased ileum causes steatorrhea.4 Type 2 is diarrhea that responds to bile acid binders in the absence of structural change, a group that includes a significant number of patients with chronic diarrhea, IBS with diarrhea (IBS-D), and microscopic colitis.4 Pale, sticky, floating, foul-smelling stool can also be the result of celiac disease caused by malabsorption of gluten. Malabsorption of bile acids resulting from use of biguanides in diabetics may cause vitamin B12 malabsorption with diarrhea and may play a role in acquired immunodeficiency syndrome (AIDS) diarrhea.4 Likewise, small intestinal bacterial overgrowth syndrome (e.g., after cholecystectomy) is associated with malabsorption. Maldigestion results from decreased bile salts as a result of congestion (e.g., cirrhosis, bile duct obstruction) and pancreatic dysfunction (e.g., chronic pancreatitis, cystic fibrosis).1 Secretory and osmotic mechanisms rather than exudative conditions are more likely to result in a very watery stool. Exudative or inflammatory conditions resulting in febrile illness or blood and pus in stool are typically associated with pain. Diverticulitis, ischemic colitis, IBD (if not pseudomembranous colitis from Clostridium difficile invasion after antibiotic exposure), and a range of other viral, bacterial, and parasitic infections represent the inflammatory causes. Cancer treatment–induced diarrhea may be considered a subcategory of the exudative or even secretory type, inclusive of radiation enteritis. Nonulcerative, microscopic colitis (usually without blood, pus, or pain) may be as common as IBD.5 Colon cancer, mastocytosis, invasive or inflammatory infections (C. difficile, cytomegalovirus, Entamoeba histolytica, tuberculosis, or ischemia is indicative1; rarely, Aeromonas and Yersinia are included in this category).3 Motility or regulation dysfunctions drive gastrointestinal contents along the tract more quickly, resulting in inadequate absorption of fluid from the colon. These transit dysfunctions may also be considered secretory-type diarrheal disorders but include such a variety of conditions as to warrant separate mention. Postsurgical complications may result in diarrhea. Surgeries with a high likelihood of this adverse effect include cholecystectomy, Whipple procedure (pancreaticoduodenectomy), Billroth II (gastrojejunostomy), gastric bypass (Roux-en-Y), ileocolonic resection, and ileocecal valve removal or compromise. Bile salt malabsorption features largely in significantly resected or diseased ileum, causing an overflow into the colon and an osmotic gradient (with secretory and osmotic features in the barrier crossing). Rapid transit may result from limited exposure to mucosal surface or from changes in the mechanical stretch receptors or neural stimuli.6 Motility disorders also include diarrhea-predominant IBS. Defined by commonly accepted Rome III diagnostic criteria for functional gastrointestinal disorders, IBS is thought to result from altered central nervous system pathways involving the limbic and paralimbic connectors, which communicate stress and receive pain signals,6 sometimes referred to as visceral hypersensitivity. Serotonin reuptake transporter has a critical effect on the increase or decrease in gastrointestinal motility through the 5-HT3 and 5-HT4 receptors.6 Although the mechanism is not yet known, these activators are thought to be involved6 and have been harnessed in medical therapy directed against diarrhea-predominant IBS. In older adults, there may be significant enteric neuron loss over time, especially in the cholinergic neurons, causing motility changes as well as a blunting of cellular and humoral immunity, autoantigen recognition, and disturbed gut flora.3 Finally, a range of functional disorders are included. A subset of patients with functional bowel disease have significant psychological disorders that are more likely to respond to psychiatric co-management. A smaller group of patients perpetuate symptoms for secondary gain or out of emotional distress.7 The initial history should include the patient’s normal stool pattern compared with the new-onset diarrhea. Charting of when the diarrhea began—whether the onset was abrupt or gradual, the course’s duration, and daily stool frequency (discrete or continuous) as well as when diarrhea occurs with respect to meals and sleep—will help frame the clinical picture. Any improvement or worsening of the condition or associated symptoms should be elicited as part of the history. Attention should be given to new-onset urgency; lower abdominal spasms, whether across the abdomen or localized to one side; relief of spasms after a movement; rectal discomfort; a sense of incomplete evacuation; or fecal incontinence. Bloody or mucopurulent exudates in the stool might reveal an inflammatory source. Greasy, bulky, rancid-smelling stool is suggestive of fat or carbohydrate malabsorption related to small bowel or pancreatic dysfunction. Whether the stool is mostly watery or unformed is also helpful in sorting out a potential secretory. Alternating patterns of diarrhea and constipation, diarrhea that awakens the patient from sleep, and any history of hemorrhoids will help determine the diagnosis. A previous history of manual disimpaction and chronic constipation or recent narcotic use might suggest overflow diarrhea in the setting of fecal impaction. It is important to ascertain symptom relief achieved in relation to diet and over-the-counter or prescription medication. Associated signs and symptoms (e.g., nausea, vomiting, dehydration, abdominal cramping, pain, fever, chills, and rash) must also be explored. Recent history of illness may point to postinfectious IBS. Foreign travel may contribute. Other pertinent information includes increased thirst, dark or concentrated urine, oliguria, dizziness, and urinary tenesmus in consideration of renal insufficiency or undiagnosed diabetes. Determination of whether unintentional weight loss (gradual or acute) has occurred is critical; this may prompt a diagnosis of Addison disease, hyperthyroidism, or malignant disease in the absence of other findings, especially in older adults. Growth retardation or delayed onset of puberty can suggest celiac disease, juvenile-onset diabetes, or cystic fibrosis. Medications are also commonly associated with diarrhea as much as 4% of the time, particularly magnesium; antihypertensives; nonsteroidal anti-inflammatory drugs (NSAIDs), associated to some extent with microscopic colitis5; antibiotics; theophyllines; and chemotherapy agents.2 Drugs highly associated with diarrhea include α-glucosidase inhibitors (acarbose), biguanides (metformin), cholinergic drugs, colchicine, digoxin, gold salts, highly active antiretroviral agents, metoclopramide, osmotic laxatives, prostaglandins, tyrosine kinase inhibitors, selective serotonin reuptake inhibitors, some immunosuppressive agents, and ticlopidine.7 Chronic prednisone use can result in hypercortisolism with symptomatic diarrhea. Finally, over-the-counter and complementary products warrant further investigation for possible adverse effect or toxicity. Allergic reactions to prescription medications and environmental exposures as well as food-related reactions are important. A dietary history should include any nutritional or dietary supplements or diet aids, especially sugar-free products that contain xylitol, sorbitol, or mannitol, which are poorly absorbed. Patients whose food sources lack vitamin B3 or niacin (from tryptophans) or who have chronic vitamin deficiency secondary to alcoholism or food deprivation may develop pellagra, which is associated with diarrhea, dementia, and dermatitis. The history should elicit previous medical conditions, in particular Addison disease, Behçet disease, common variable immunodeficiency disease, cystic fibrosis, celiac disease with signs of dermatitis herpetiformis or osteoporosis, diabetes mellitus type 1 or acquired diabetes mellitus, human immunodeficiency virus (HIV) infection or AIDS, hyperthyroidism or potentially overmedicated hypothyroidism, ischemia or renal blood flow difficulties, pancreatic insufficiency or other pancreatic conditions, protein-losing enteropathies, short bowel syndrome, and Zollinger-Ellison syndrome, a pancreatic neuroendocrine tumor in which 90% of patients have peptic ulcer disease.8 Personal history of intestinal lymphoma, carcinoid, or other gastrointestinal tumors or prior radiation therapy is important because radiation enteritis can result in diarrhea many years later. Gastric bypass, Billroth II, or a Whipple procedure and associated complications, such as fistulas, blind loops, and strictures, may contribute to bacterial overgrowth and chronic mucosal surface changes, leading to diarrhea. Obstetric injury to the anal sphincter may be especially pertinent if there is fecal incontinence, which is perceived as diarrhea. A family medical history of the same or other chronic gastrointestinal conditions or cancers would be clinically relevant. A social history of tobacco product use, alcohol abuse, or illicit drug or recently unsupervised narcotic use may contribute to diarrhea. Smoking has been shown to increase the risk and earlier presentation of lymphocytic and collagenous colitis.5 Diarrhea can be associated with weight loss medications, in particular amphetamines and caffeinated products, such as stimulating energy drinks and tea preparations. Finally, laxative abuse and other disturbed eating, such as bulimia, might raise concern for self-injurious behavior. Munchausen syndrome, factitious diarrhea for secondary gain, malingering, and hypochondriasis are also seen. Situational stress, heightened anxiety, and panic attacks, which can be seen in association with depression, might contribute to a change in bowel pattern. The physical examination includes temperature and orthostatic vital signs (blood pressure and heart rate [lying, sitting, and standing]) to assess volume depletion. The patient’s mental status should be noted along with other signs or symptoms of wasting, including weight loss. Dry mucous membranes, decreased skin turgor, and absent jugular venous pulsation would suggest significant dehydration. A close assessment should be done for skin pallor or hair thinning in patients with anemia, rashes, flushing, and warmth; adrenocorticotropic hormone (ACTH)–related darkening of palmar creases and other sites, as in Addison disease; dermatographia (mast cell disease); icteric conjunctiva in advanced liver disease; evidence of exophthalmos and eyebrow thinning (hyperthyroidism); eye redness and pain of uveitis (Whipple disease, episcleritis or dry eyes associated with IBD); subcutaneous bleeding resulting from lack of vitamin K or prolonged prothrombin time (cirrhosis); and erythema nodosum (ulcerative colitis). The head and neck should be assessed for evidence of immunocompromise, such as lymphadenopathy or oral leukoplakia in those undergoing cancer treatment or AIDS patients, macroglossia (in rare cases of amyloidosis), or mouth ulcers in IBD, as well as thyromegaly. Carotid bruits may indicate arterial flow disease. Flushing might relate to carcinoid syndrome or mastocytosis. A cardiovascular examination is indicated to exclude the cardiac complications associated with some illnesses, such as tachycardia in hyperthyroidism. Chest findings would be unusual except in suggesting systemic or metastasized diseases. Wheezing might signify a carcinoid tumor. During the abdominal examination, particular care should be taken in noting abdominal scars, visible distention, and audible activity of the bowel including a succussion splash, which may provide evidence of delayed gastric emptying. In addition, an indication of impaired arterial flow would be seen in bounding abdominal pulses, heard as bruits. For completion of the examination, palpation should be performed for tenderness, rigidity, rebound, guarding, masses, organomegaly, or ascites. Gauging anorectal sphincter tone as well as performing a digital rectal examination for masses, fecal impaction, or bleeding would be included. In the female patient with lower abdominal symptoms, a pelvic examination is imperative. Tremor may be a sign of hyperthyroidism. Joint pains might point to IBD, reactive arthritis after enteritis such as Reiter syndrome, or a vitamin D and calcium deficiency resulting from malabsorption. Distal extremity edema suggests interstitial fluid shift, possibly from extravascular fluid leak or protein malabsorption as a result of chronic malnutrition or protein-losing enteropathy. For the patient who has mild, afebrile, acute diarrhea, diagnostic evaluation is not usually indicated. These brief episodes are typically viral or food-borne illnesses, are self-resolving, and require little or no intervention (Box 132-1). If an infectious source is suspected, stool for fecal leukocytes, ova, and parasites; a stool culture; and sensitivity testing are necessary. In the absence of an infectious cause, plain abdominal x-ray examination of the kidneys, ureters, and bladder (KUB), flat and upright, is necessary if a small bowel obstruction or stool impaction with overflow incontinence is suspected. Emergent indications for upper endoscopy with small bowel follow-through include small bowel torsion causing partial or full obstruction, possibly resulting from colitis-related strictures, bowel perforation, toxic megacolon, or ileus. If diarrhea continues after 2 weeks and the suspected cause is noninfectious, then colitis, diverticulitis, pancreatitis, irritable bowel, and IBD would lead the list of differential diagnoses. The patient’s weight is a key indicator, especially in persistent or chronic cases. A complete blood count (CBC) with differential revealing an elevated white blood cell count may indicate general inflammation or infection; low hematocrit and hemoglobin would indicate anemia, potentially as a result of acute blood loss, which may corroborate a positive stool guaiac test result over time. A complete metabolic panel including liver function would reveal electrolyte or enzyme abnormalities. A compromise in kidney function would suggest dehydration or concomitant illness is a factor. In patients with a temperature above 38.8° C (102° F), bloody diarrhea, abdominal pain, more than six unformed stools in a 24-hour period, profuse watery diarrhea, and dehydration or in patients who are frail or older, immunocompromised, or toxic appearing, a stool sample should be sent for C. difficile toxin A or B analysis, with secondary consideration of IBD.
Diarrhea, Noninfectious
Definition and Epidemiology
![]() Prompt medical evaluation is indicated if diarrhea is associated with fever, abdominal pain, dehydration, or bloody stool.
Prompt medical evaluation is indicated if diarrhea is associated with fever, abdominal pain, dehydration, or bloody stool.
Pathophysiology
Clinical Presentation
Physical Examination
Diagnostics
Acute Diarrhea
Diarrhea, Noninfectious
Chapter 132