Chapter 50 Diabetic emergencies
Diabetes mellitus is due to an absolute or relative deficiency of insulin. The sustained effect of poor glycaemic control results in a wide array of end-organ damage as a consequence of small- and large-vessel pathology. Mortality and morbidity are related to the progress of this damage but often there are acute metabolic deteriorations that can be life-threatening. Diabetic ketoacidosis (DKA) and hyperosmolar hyperglycaemic state (HHS) are two of the most common acute complications of diabetes, both accompanied by hyperglycaemia. The pathophysiologic changes that occur in both disease states represent an extreme example of the superfasted state. Coma may also result from severe hypoglycaemia due to overtreatment, usually with insulin.
DIABETES MELLITUS
TYPE 1
Insulin-dependent diabetes mellitus (IDDM) has a peak incidence in the young, rising from 9 months to 14 years and declining thereafter. In 25% of patients the presentation is with ketoacidosis, especially in those under 5 years of age.1 Usually the fasting plasma glucose is > 7.8 mmol/l and glucose and ketones may be present in the urine. In the asymptomatic patient with equivocal fasting plasma glucose an impaired glucose tolerance test may be demonstrated.
TYPE 2
Non-insulin-dependent diabetes mellitus (NIDDM) is prevalent in the elderly but can occur at any age. Truncal obesity is a risk factor and there is ethnic variation in susceptibility. Diagnosis is often delayed and may be incidental from blood or urine sugar screening.2 It may present with classical symptoms, as a diabetic emergency, with complications of organ damage or vascular disease.
EPIDEMIOLOGY
Diabetes mellitus affects about 6% of the world’s population and is set to rise to 300 million sufferers by 2025.3 Most of these (97%) will have type 2 diabetes but the resources required to treat the complications of type 1 diabetes are such that health-care costs are equivalent between the two groups. The annual incidence of DKA is 4.6–8 episodes per thousand patients with type 1 diabetes and in the USA it has been estimated that about $1 billion is spent each year in treating DKA. HHS represents approximately 1% of primary admissions to hospital with diabetes as compared with DKA. An interplay of both genetic and environmental factors contributes to disease development. In type 1 diabetes there is some evidence for genetic susceptibility, but environmental factors play a greater part. It varies across race and regions, being highest in northern Europe and the USA and lowest in Asia and Australasia.4 Genetic factors in type 2 diabetes are evidently crucial, with a concordance between monozygotic twins approaching 100%.
PATHOGENESIS
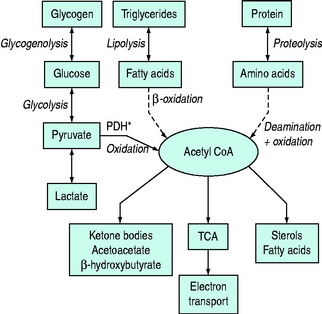
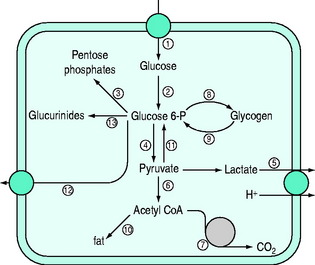
Normal carbohydrate metabolism depends upon the presence of insulin (Figure 50.1). However, different tissues handle glucose in different ways; for example, red blood cells lack mitochondria and therefore lack pyruvate dehydrogenase and the enzymes involved in β-oxidation, whereas liver parenchymal cells are able to perform the full range of glucose disposal (Figure 50.2). Both DKA and HHS result from a reduction in the effect of insulin with a concomitant rise in counterregulatory hormones such as glucagon, catecholamines, cortisol and growth hormone. Hyperglycaemia occurs as a consequence of three processes: (1) increased gluconeogenesis; (2) increased glycogenolysis; and (3) reduced peripheral glucose utilisation. The increase in glucose production occurs in both the liver and the kidneys as there is a high availability of gluconeogenic precursors such as amino acids (protein turnover shifts from balanced synthesis and degradation to reduced synthesis and increased degradation). Lactate and glycerol also become available due to an increase in skeletal muscle glycogenolysis and an increase in adipose tissue lipolysis respectively. Lastly, there is an increase in gluconeogenic enzyme activity, enhanced further by stress hormones. Whilst hepatic gluconeogenesis is the main mechanism for producing hyperglycaemia, a significant proportion can be produced by the kidneys.5 What is unclear is the temporal relationship of these changes, although an increase in both catecholamines and the glucagon/insulin ratio are early features.6
By contrast HHS does not share the ketogenic features of DKA and it is of interest in what ways these two conditions differ. Reduced levels of FFAs, glucagon, cortisol and growth hormone have been demonstrated in HHS relative to DKA, although this is by no means a consistent observation. However, the presence of higher levels of C-peptide in HHS (with lower levels of growth hormone) relative to DKA suggests there is just enough insulin present in HHS to prevent lipolysis but not enough to promote peripheral glucose utilisation.7
Interestingly, hyperglycaemia, with or without ketoacidosis, leads to a significant increase in proinflammatory cytokine production, which resolves when insulin therapy is commenced.8 This has led others to postulate a wider beneficial anti-inflammatory effect attributable to insulin therapy.
CLINICAL PRESENTATION
DKA and HHS represent the two extremes of presentation due to the absolute or relative deficiency of insulin. However, up to one-third of cases can present with mixed features.9 DKA develops over a shorter time period whereas HHS appears more insidiously (Table 50.1). Polyuria, polydipsia and weight loss are experienced for a variable period prior to admission and, in patients with DKA, nausea and vomiting are also common symptoms. Abdominal pain is commonly seen in children and occasionally in adults and may mimic an acute abdomen. Dehydration presents with loss of skin turgor, dry mucous membranes, tachycardia and hypotension. Mental obtundation occurs more frequently in HHS than DKA as more patients, by definition, are hyperosmolar and the presence of stupor or coma in patients who are not hyperosmolar requires consideration of other potential causes for altered mental status.10 However, loss of consciousness is not a common presentation with DKA or HHS (< 20%). Most hospitalisations are caused by infection (29%) or non-compliance with medication (17%) in previously diagnosed diabetics; however, in some patients it is the first presentation with undiagnosed diabetes (17%).11 Given that there is a high likelihood of concurrent infection, most patients have a normal or low temperature and most patients also have a leukocytosis, whether there is infection present or not.
Table 50.1 Comparison of DKA and HHS
| Presentation | DKA | HHS |
|---|---|---|
| Prodromal Illness | Days | Weeks |
| Coma | ++ | +++ |
| Blood glucose | ++ | +++ |
| Ketones | +++ | 0 or + |
| Acidemia | +++ | 0 or + |
| Anion Gap | ++ | 0 or + |
| Osmolality | ++ | +++ |
| Typical deficits | ||
|---|---|---|
| Total water (litres) | 6 | 9 |
| Water (ml/kg) | 100 | 100–200 |
| Na+ (mEq/kg) | 7–10 | 5–13 |
| Cl− (mEq/kg) | 3–5 | 5–15 |
| K+ (mEq/kg) | 3–5 | 4–6 |
| PO4 (mEq/kg) | 5–7 | 3–7 |
| Mg2+ (mEq/kg) | 1–2 | 1–2 |
| Ca2+ (mEq/kg) | 1–2 | 1–2 |
HYPEROSMOLAR HYPERGLYCAEMIC SYNDROME
HHS is more often seen in patients with type 2 diabetes and the dominant feature is hyperosmolarity (> 320 mosmol/kg). HHS is typically observed in elderly patients with NIDDM, although it may rarely be a complication in younger patients with IDDM, or those without diabetes following severe burns,12 parenteral hyperalimentation, peritoneal dialysis or haemodialysis. Patients receiving certain drugs, including diuretics, corticosteroids, β-blockers, phenytoin and diazoxide, are at increased risk of developing this syndrome. HHS may be caused by lithium-induced diabetes insipidus.13 Not only may mental obtundation occur but occasionally focal neurological features or seizures are present.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree