Mary E. Wood Diabetes mellitus is the most common metabolic disorder seen in primary care and a leading cause of cardiovascular disease (CVD), renal failure, blindness, and nontraumatic lower limb amputation. The American Diabetes Association (ADA) offers this definition: “Diabetes is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both.”1 The World Health Organization states: “Diabetes is a condition primarily defined by the level of hyperglycemia giving rise to risk of microvascular damage (retinopathy, nephropathy, and neuropathy). It is associated with reduced life expectancy, significant morbidity due to specific diabetes-related microvascular complications, increased risk of macrovascular complications (ischemic heart disease, stroke, and peripheral vascular disease), and diminished quality of life.”2 The total prevalence of diabetes in the United States as of 2012 was 29.1 million (9.3% of the population). Among adults aged 20 years and older, the prevalence of diabetes is slightly higher in men than in women (13.6% versus 11.2%). The prevalence of diabetes increases with age and is higher in Asian Americans (9.0 %), Hispanics (12.8%), non-Hispanic blacks (13.2%), and American Indians/Alaska Natives (15.9%) than in non-Hispanic whites (7.6%). Of the 29.1 million Americans with diabetes, 21.0 million have been diagnosed and 8.1 million remain undiagnosed.3 An estimated 86 million Americans aged 20 and older have prediabetes, with elevated glucose levels that do not meet the criteria for the diagnosis of diabetes. The prevalence of prediabetes is similar for non-Hispanic whites (35%), non-Hispanic blacks (39%), and Hispanics (38%).3 Among individuals with prediabetes, healthy diet, regular exercise, and weight loss have been shown to prevent or delay the progression to diabetes.4 Given this, an important focus of primary care is prevention of type 2 diabetes. Screening is recommended for early diagnosis (Box 206-1). An estimated 90% to 95% of people in the United States with diabetes have type 2 diabetes (previously known as adult-onset diabetes, type II diabetes, or non–insulin-dependent diabetes mellitus). Fewer than 10% have type 1 diabetes (formerly known as juvenile-onset diabetes, type I diabetes, or insulin-dependent diabetes)1,3 (Box 206-2). Type 1 diabetes typically begins in childhood, adolescence, or early adulthood but can manifest at any age. Type 2 diabetes, which is strongly linked to obesity, was in the past diagnosed in middle-aged and older individuals but is now more commonly developing in childhood and adolescence. The incidence of both type 1 and type 2 diabetes is increasing, and the disease is occurring at an earlier age.5 The long-term complications of diabetes are a result of the microvascular and macrovascular damage to target end organs: the eyes, kidneys, heart, blood vessels, and nerves. Direct medical costs for diabetes care in 2012 were an estimated $17 billion. Indirect costs for disability and premature death were $69 billion.3 To reduce the devastating effects of this disease, prevention, early detection, and aggressive treatment of the long-term complications of diabetes are essential. Type 1 and type 2 diabetes share the features of hyperglycemia and an increased risk for vascular and neuropathic complications. Physiologically, they are two distinct diseases. Type 1 diabetes is caused by the autoimmune destruction of the beta cells within the islets of Langerhans in the pancreas in a genetically predisposed individual. This results in insulinopenia and the lifelong dependence on exogenous insulin. Beta cell destruction is typically more rapid in infants and children and more gradual in adults. The majority of individuals with type 1 diabetes will test positive for the presence of antibodies (islet cell autoantibodies, insulin autoantibodies, GAD65 autoantibodies, or autoantibodies to tyrosine phosphatases IA-2 and IA-2β).5 The surgical removal of the pancreas (e.g., Whipple procedure or pancreatectomy) results in type 1 diabetes with the added challenge created by the lack of glucagon secretion from the alpha cells. Insulin is required for most of the body’s tissues to take up glucose as the preferred source of energy. Thus, insulin deficiency impairs the uptake of glucose, resulting in the search for energy elsewhere. Fats and proteins are broken down, and counterregulatory hormones trigger glycogenolysis. Inadequate insulin leads to hyperglycemia. The pathophysiologic mechanism of type 2 diabetes is more obscure. Hallmarks of this disease have been called the “triumvirate” of decreased glucose uptake (insulin resistance), increased hepatic glucose production, and impaired insulin secretion. A new model of type 2 diabetes pathophysiology has been described by DeFronzo and called “the ominous octet,” in recognition of the fact that there are eight mechanisms that contribute to type 2 diabetes.6 The additional mechanisms include increased glucagon secretion, increased glucose reabsorption by the kidney, increased lipolysis, decreased incretin effect, and neurotransmitter dysfunction in the brain. Fasting hyperglycemia results from increased hepatic glucose production in the impaired first phase of insulin secretion. Postprandial hyperglycemia is caused by the decreased uptake of glucose in the skeletal muscles. In response to the elevated blood glucose levels, the insulin pathways become resistant to hormonal impulses, resulting in hyperinsulinemia. Insulin resistance by definition is the decreased sensitivity of tissue to glucose uptake with normal concentrations of insulin.1 As hyperglycemia increases, so does insulin resistance. The body is able to adapt and to maintain homeostasis for a while, but as hyperglycemia progresses, diabetes occurs. As the degree of glucose intolerance advances, hyperglycemia results from the insufficient insulin produced by the beta cells. The natural progression of type 2 diabetes includes normal glucose values as insulin resistance begins. Increased insulin secretion compensates for the resistance. As insulin resistance worsens, postprandial glucose values begin to rise. Later, insulin secretion begins to wane and fasting glucose levels start to climb. Placing the patient along this continuum will guide treatment decisions. For example, early in type 2 diabetes, treatment should aim to improve sensitivity to insulin. Later, treatment may require the enhancement of insulin secretion or endogenous insulin. Primary insulin resistance, a defect in the target cells of insulin receptors and postreceptors, results in altered insulin action and sensitivity. The onset of insulin resistance can occur with hyperinsulinemia, in the fasting or fed state. The fed state is the time associated with insulin secretion after food intake to carbohydrate metabolism and synthesis of fat and protein. As insulin resistance proceeds, glucose transportation or use of glucose in the cell is altered. Secondary resistance is caused by hormones or abnormal physiologic states (e.g., puberty, pregnancy, advanced age). Other factors associated with the development of insulin resistance include a high-fat diet, sedentary lifestyle, smoking, and weight gain. Metabolic stress, as with illness and obesity, increases insulin resistance. Many patients who have prediabetes or type 2 diabetes also have the metabolic syndrome. The metabolic syndrome is a group of metabolic components, synergistic in nature, that contribute to CVD. These components include abdominal obesity, insulin resistance and hyperglycemia, elevated triglyceride and low high-density lipoprotein (HDL) levels, hypertension, and a pro-inflammatory state. Weight loss, improved glycemic control, lipid management, and improved blood pressure may decrease the significance of this syndrome (see Chapter 212). An individual with untreated type 1 diabetes will typically be seen after a brief period of profound symptoms. Polyuria, polydipsia, polyphagia, weight loss, blurred vision, and fatigue are overt signs of diabetes. Later, as the glycosuria increases, nausea, vomiting, abdominal pain, rapid shallow breathing, hypotension, and dehydration are signs of ketoacidosis (see the section Acute Complications of Diabetes). Medical care is essential. The patient with type 2 diabetes may have no symptoms or only subtle symptoms that may persist for weeks, months, or even years before detection. Unfortunately, during this time, the vascular and neuropathic complications may begin to develop and progress before the diagnosis is made.4 The symptoms include polyuria, polydipsia, blurred vision, fatigue, slowly healing wounds, and frequent infections. Some individuals may experience polyphagia and weight loss or numbness or tingling of feet and hands. At the time of diagnosis of diabetes, the physical examination focuses on dehydration, weight loss, and precipitating causes, such as illness, infection, or stress. The patient may appear dry and flushed. The skin, eyes, heart, and lungs should be assessed. The thyroid should be palpated because type 1 diabetes is associated with thyroid disorders. In patients with new type 2 diabetes, examination should be performed for early evidence of vascular and neuropathic complications as well as for persistent infections. The purpose of periodic examination of patients with known diabetes is threefold: (1) to evaluate blood glucose control because poor control leads to end-organ complications; (2) to assess for the presence or progression of end-organ damage; and (3) to assess for associated diseases, such as other autoimmune disorders and cardiovascular risk factors. Annual examinations are comprehensive. Periodic visits, every 3 months for patients with type 1 diabetes and those with type 2 diabetes who have one or more complications, should be conducted to assess end-organ involvement and glycemic control. Visits can be stretched to every 6 months if the individual with type 2 diabetes is stable and in control. Each examination should include weight and blood pressure measurements, a review of glycemic control, and evaluation of target end-organ damage4 (Box 206-3). The diagnostic criteria for diabetes are based on the glucose threshold above which the risk of retinopathy is increased.1 In a patient exhibiting significant symptoms of hyperglycemia, a random plasma glucose of 200 mg/dL or higher is diagnostic for diabetes. In an individual without symptoms, a second confirmatory test is recommended. In July 2009, an International Expert Committee recommended the use of hemoglobin A1c (HbA1c) as a diagnostic tool. An HbA1c of 6.5% or higher, a fasting plasma glucose of 126 mg/dL or higher, or a 2-hour plasma glucose level of 200 mg/dL or higher during an oral glucose tolerance test fulfills the diagnostic criteria for diabetes1 (Table 206-1). TABLE 206-1 Diagnostic Criteria for Diabetes and Prediabetes HbA1c is a nonfasting test and has the same relationship to risk of retinopathy as do the other tests. Because of the chance of laboratory error, this test should also be repeated in the absence of classic symptoms of hyperglycemia. HbA1c results may be inaccurate in patients with anemia or other hemoglobinopathies and during pregnancy. If necessary, a C-peptide level can be helpful in distinguishing type 1 from type 2 diabetes. Markers of type 1 diabetes include islet cell autoantibodies, insulin autoantibodies, and GAD65 autoantibodies. Delivering the diagnosis of diabetes to the patient should include not only the message that this is a serious disease for which there is no cure but also the positive encouragement that the patient will learn to manage the disease and be able to continue to work and to do the things that bring enjoyment. Inclusion of supportive family members or friends in this discussion is helpful. The diagnosis of diabetes is straightforward in a patient with polyuria and polydipsia. The differential diagnosis is limited to type 1 diabetes, type 2 diabetes, and diabetes insipidus. If hyperglycemia and glycosuria are absent and diabetes insipidus is excluded, consider hyperthyroidism (check triiodothyronine [T3], thyroxine [T4], and thyroid-stimulating hormone levels) or hyperparathyroidism (check parathyroid hormone and serum calcium levels). Secondary causes of diabetes should always be considered. These include excess of counterregulatory hormones (Cushing syndrome, pheochromocytoma, and acromegaly); significant hypokalemia caused by glucose intolerance; hyperaldosteronism or diuretic use; and destruction in the pancreatic islet from pancreatitis (caused by alcoholism or gallbladder disease), hemochromatosis, or drug-induced islet cell injury. In addition, infection or medication may cause glucose intolerance. The differentiation between type 1 and type 2 is important for therapeutic purposes because patients with type 1 diabetes must receive insulin daily. Treatment of diabetes includes a healthy diet, regular exercise, medication, monitoring, self-care education, and periodic follow-up with the primary care provider and the diabetes care team. The combination of these therapies will aid the patient in achieving the best glycemic control possible without undue burden or adverse effects (Table 206-2). TABLE 206-2 Glycemic Control Targets for Nonpregnant Adults Nutritional therapy is essential in management of both type 1 and type 2 diabetes. A registered dietitian is crucial in helping to individualize a meal plan and to teach the patient and family about healthy nutrition.7,8 The goal of nutritional therapy for diabetes and prediabetes is the development of the meal plan, balancing insulin with food intake and activity to achieve glycemic control. The nutrition goal for patients with type 1 diabetes is to promote normal growth and development during childhood, adolescence, pregnancy, and lactation; to balance energy intake and expenditure; and to achieve near-normal blood glucose levels. Matching insulin doses to carbohydrate intake is necessary. For patients with type 2 diabetes, the nutrition goals are the achievement and maintenance of a healthy weight, adequate blood pressure control and lipid levels, and good glycemic control (Box 206-4). To promote recovery from a severe illness, special dietary adjustments may be necessary and can be individualized with the help of a registered dietitian. Exercise and physical activity have been shown to improve glycemic control.9 Exercise causes increased glucose uptake in skeletal muscles as well as improved insulin sensitivity. All of the other benefits of exercise for the heart, lungs, and state of mind help reduce the risk of diabetes complications. It is important for the individual with type 1 diabetes to make appropriate adjustments in food intake and/or insulin doses to balance the effects of exercise. Postexercise hypoglycemia or hyperglycemia may occur. The exercise lag effect refers to the low blood glucose concentration that may happen many hours after exercise. To prevent hypoglycemia during exercise, the individual must balance activity with adequate food and appropriate insulin doses. High blood glucose levels after exercise may result from decreased circulating insulin and glucose uptake and increased hormonally regulated hepatic glucose. In the setting of exercise without adequate circulating insulin, ketosis is increased as fatty acids are broken down for energy, resulting in higher blood glucose levels and possible ketosis.9 In the individual with type 2 diabetes, exercise decreases insulin resistance and increases glucose uptake. Increased insulin sensitivity, contributing to the exercise lag effect, can last up to 48 hours after exercise. Thus, food intake and medication, especially insulin, need to be adjusted for the activity or exercise. A reduction in the insulin that is peaking at the time of exercise and perhaps the basal insulin as well may be necessary. In the patient with type 2 diabetes, it would be desirable to decrease the medication rather than increasing food consumption so as to promote weight loss.9 Exercise has been shown to prevent or delay the progression from prediabetes to diabetes. Guidelines for exercise in diabetes are reviewed in Box 206-5. Insulin, an anabolic hormone produced by the beta cells of the pancreas, plays a vital role in metabolism. Insulin therapy is the lifesaving treatment for type 1 diabetes. Insulin is also used by patients with type 2 diabetes who have persistent hyperglycemia despite lifestyle changes and oral and/or noninsulin injectable diabetes agents. An individual with type 2 diabetes who begins to take insulin does not then have type 1 diabetes. Rather, he or she now has insulin-treated type 2 diabetes. Physiologic secretion of insulin is biphasic: basal and prandial. The basal phase inhibits glycogenolysis and gluconeogenesis and maintains glucose in a steady state. The prandial phase controls the initial glucose load and reuptake. Early morning hyperglycemia caused by counterregulatory hormones (known as the dawn phenomenon) is controlled by basal insulin, and postmeal glucose spikes are controlled by prandial insulin. Insulin therapy should mimic this response. There are a number of insulin products available that may be prescribed to tailor an individualized regimen for each patient, with the goal of optimal glycemic control while allowing a flexible lifestyle. Human insulin is derived synthetically from recombinant DNA (Humulin [Eli Lilly], Novolin [Novo-Nordisk]). Human insulin is available in regular, NPH, and 70/30, a premixed combination of 30% regular and 70% NPH. Humulin 50/50, pork-derived regular and NPH insulins, and Lente and Ultralente insulins are no longer available. The first inhaled insulin (Exubera) was briefly marketed but withdrawn by the manufacturer because of disappointing sales. A new insulin inhalation powder (Afrezza) received U.S. Food and Drug Administration (FDA) approval in June 2014 and was initially marketed in early 2015. This is a rapid-acting insulin approved for use in adults. Individuals who use Afrezza must also take subcutaneous basal insulin daily. Side effects include hypoglycemia, cough, and throat pain. Inhaled insulin is not recommended for patients who smoke. Five insulin analogues made of recombinant human insulin are available. Three are rapid acting (lispro, aspart, glulisine), and two are long acting or peakless (glargine and detemir). The three rapid-acting insulin products are used as prandial and correction insulin, and the peakless insulins provide true basal insulin coverage. Premixed insulin combinations are available for convenience. Individualized doses of basal and prandial insulin offer the best and most physiologic approach to tight glycemic control, with fewer episodes of hypoglycemia. Individuals with type 1 diabetes are truly dependent on insulin for survival. Simplistically, type 1 diabetes is the absence of one hormone: insulin. The replacement of that hormone to mimic normal physiology is challenging. Individuals with type 1 diabetes are sensitive to insulin, with a dramatic response to too much insulin (hypoglycemia) or too little (hyperglycemia or diabetic ketoacidosis [DKA]). The patient must appreciate that daily insulin is essential. The most physiologic regimen is basal/bolus insulin. This can be accomplished with multiple daily injections or with an insulin pump, also known as continuous subcutaneous insulin infusion. Exogenous insulin requirements vary from one patient to another and, for an individual, from day to day. Physiologic insulin secretion for adults who do not have diabetes is approximately 20 to 40 units/day.10 Thus, most adults with type 1 diabetes will need to inject approximately this much insulin every 24 hours. One unit of any kind of insulin will cause the same reduction in blood glucose concentration in a given individual for the duration of that type of insulin. For example, 1 unit of a rapid-acting insulin may drop one patient’s blood glucose level 60 mg/dL in 2 to 3 hours, and 1 unit of a long-acting insulin will reduce the glucose level 60 mg/dL for over 12-24 hours. A patient who is much more resistant to insulin will experience a drop of only 10 mg/dL in glucose concentration in 2 to 3 hours after 1 unit of rapid-acting insulin and a drop of 10 mg/dL for 24 hours after 1 unit of basal insulin. When insulin treatment is initiated, body weight, morphologic development (obese versus muscular), age (adolescent versus elderly), and activity (sedentary versus athletic) must be considered. The type of insulin prescribed depends on the patient’s needs and the provider’s preference. Therapy should begin with conservative starting doses and continuously titrated to achieve the desired blood glucose levels. On the basis of the patient’s weight, an initial total daily dose (TDD) may be calculated as 0.5 unit/kg. The TDD is then divided into basal and bolus doses. Fifty percent of the TDD is basal insulin, and 50% is divided among the mealtime doses. Frequent premeal and postmeal blood glucose monitoring will guide dose adjustments. Glargine and detemir, true long-acting insulins, are typically given once daily, often at bedtime, and last for up to 24 hours, mimicking the basal secretion of insulin.10 Basal insulin may be given at a different time of day, if it is more convenient for the patient, to maintain a consistent routine. Large doses may be divided into twice-daily injections given 12 hours apart. Some individuals notice a peak from their basal insulin, so they may split their dose into two daily injections to minimize this. The dose of basal insulin is not determined with a sliding scale, so as not to make a decision with 24-hour implications based on a single blood glucose value. Rather, the dose of basal insulin should be adjusted every 2 or 3 days until the fasting blood glucose level is less than 110 mg/dL. The basal insulin should be increased by increments of 2 to 5 units in an obese, insulin-resistant individual or by 1 to 2 units in an insulin-sensitive patient with a thin body frame or in patients with frequent episodes of hypoglycemia.10 If the fasting glucose concentration is consistently lower than 80 mg/dL, the dose of basal insulin should be reduced. To prevent high postprandial glucose levels, a rapid-acting insulin analogue should be injected just before, during, or immediately after the meal. A low premeal blood glucose level, unpredictable food intake, and delayed gastric emptying are common reasons for waiting until after the meal to inject the bolus dose. Most often, mealtime insulin is injected 10 minutes before eating. A bolus dose with meals allows more flexibility in mealtimes, prevention of postprandial hyperglycemia, and fewer episodes of hypoglycemia.10 Initially, mealtime doses are The goal of the diabetes plan is to attain glycemic control with appropriate insulin doses but without symptoms of hypoglycemia or hyperglycemia. Intensive insulin therapy is defined as four or more insulin injections each day or use of an insulin pump. This comprehensive strategy requires collaboration among the individual patient and family, the health care provider or diabetes specialist, and the diabetes education team of a nurse educator and dietitian. The more the patient knows about diabetes, the better and safer the diabetes management will be. Hypoglycemia is the most serious side effect of insulin. Fear of hypoglycemia prevents some individuals from embracing intensive insulin therapy. To prevent both hypoglycemia and hyperglycemia, factors such as exercise and activity, meal composition, mealtimes, sleep patterns, illness, and psychological well-being must be considered in adjusting insulin doses. The most important tool for control of day-to-day variations and prevention of hypoglycemia is home blood glucose monitoring. For a patient using an insulin pump, co-management with an endocrinologist and diabetes care and education team is strongly recommended. Intensive insulin therapy is not appropriate for all patients, including those with hypoglycemia unawareness, those who are poorly motivated and unwilling to monitor their blood glucose levels frequently, older adults, and those who have a limited life expectancy.10 Basal/bolus insulin treatment is complex and more expensive than a twice-daily regimen of regular and NPH insulin or premixed insulin. This treatment regimen is an alternative for patients who cannot or will not take multiple daily injections. The patient using twice-daily insulin must eat meals on a schedule and be aware of the risk of nocturnal hypoglycemia. Regular insulin may be used as a less expensive alternative to a rapid-acting analogue but does not offer the immediate mealtime coverage, resulting in hyperglycemia postprandially and then hypoglycemia before the next meal. The four prepared insulin mixtures—Humalog 75/25, NovoLog 70/30, and mixtures of NPH and regular 70/30 (Novolin, Humulin)—offer ease and convenience for the individual. Dose adjustments, however, change both the short-acting and longer-acting insulin. Insulin products are available in both a vial (to be drawn into a syringe) and a prefilled, disposable pen. A prescription for insulin must be accompanied by a second prescription for either syringes or insulin pen needles. Syringes are available in several different sizes, and needles come in several different lengths. NPH and regular insulin may be mixed in one syringe and injected together. Glargine and detemir cannot be mixed with another insulin (Box 206-6). New concentrated insulin products, allowing the injection of a smaller volume, include Humalog U-200 and glargine U-300 (Toujeo). These are available only in pens, and doses are prescribed in actual units of insulin. Humulin Regular insulin U-500 has been available since 1997 and is known to behave differently than U-100 regular insulin. It is not yet available in a pen, so caution is necessary in prescribing to prevent errors (see later). Frequent glucose monitoring is recommended after a change in insulin concentration, because dose adjustments may be necessary. Requirements for insulin increase during illness, surgery, and growth spurts and in patients with ketoacidosis. Insulin absorption from subcutaneous tissues varies about 25% among patients. The practitioner should be aware of a possible “honeymoon” phase in the patient with newly diagnosed type 1 diabetes with recovering beta cell function. Insulin requirements may decrease to 0.2 to 0.5 unit/kg body weight per day during this short-term phase.4 Individuals with type 2 diabetes are not dependent on insulin but may require insulin for optimal glycemic control. This is common in a patient after several years of diabetes that has been well controlled with diet, exercise, and oral diabetes medications and may represent beta cell exhaustion. Patients must appreciate that this does not represent a “failure” on their part but rather is the natural progression of the disease. Encouragement to accept insulin as the most appropriate treatment of this phase of their diabetes, rather than to fear injections, is a useful strategy when insulin treatment is recommended (Box 206-7). Insulin may be added to one or more oral diabetes medications or may replace oral medications. Because additional insulin may contribute to weight gain, it is essential that the patient understand that a healthy diet, regular exercise, and weight loss remain the cornerstones of diabetes treatment. The introduction of a medication does not take the place of lifestyle modification. As a first step, a single bedtime injection of intermediate- or long-acting insulin may be added to the oral medications to control fasting hyperglycemia and to provide basal insulin throughout the day. Basal insulin plus an insulin secretagogue for prandial glucose control works well for some. If the individual’s glucose level remains above the targets after a few weeks, a rapid-acting insulin analogue can be added at mealtimes. Regular insulin may be used as an alternative but does not offer immediate mealtime coverage, so it may result in hyperglycemia postprandially, and then hypoglycemia before the next meal. Use of lispro, glulisine, or aspart before meals allows more flexibility, better coverage of hyperglycemia, and fewer episodes of hypoglycemia.10 Initially, the analogue could be administered as a fixed dose with each meal or in different doses for low-carbohydrate and high-carbohydrate meals. When ready, the patient could be taught to count grams of carbohydrate and to calculate a more exact dose based on an individualized carbohydrate/insulin ratio. Local reactions at the injection site are the most common form of allergic reaction to insulin. Delayed hypersensitivity may also occur but remains in the area of injection. The use of synthetic or purified insulin has decreased both local and systemic reactions. Occurrence of reactions may be secondary to improper injection technique, injection of cold insulin, or preservatives. If systemic reactions do occur, the individual may require desensitization (the process of slowly reintroducing the allergy-inducing insulin at minute doses until the body no longer has an allergic response). Pramlintide (Symlin) is a synthetic analogue of human amylin and may be added to insulin treatment in both type 1 and type 2 diabetes. Amylin is a hormone produced in the pancreas and co-secreted with insulin. Amylin becomes deficient as beta cells are destroyed. Its role in diabetes treatment is to reduce the amount of glucose in the bloodstream by reducing the amount of food consumed. The release of amylin leads to a decrease in hepatic glycolysis and a slowing of gastric emptying into the small intestine, thereby increasing satiety. The results are decreased glucagon secretion and decreased postprandial glucose spikes. Available now only in a disposable, multidose pen, pramlintide is given by subcutaneous injection 10 to 15 minutes before meals, starting at a low dose of 15 to 60 µg (type 1) and 30 to 120 µg (type 2) and titrating slowly upward. The dose of mealtime insulin should be decreased by 30% to 50% and taken toward the end of the meal. Side effects include severe hypoglycemia, nausea, anorexia, and gastrointestinal distress. Therefore, it is important to titrate slowly, particularly if side effects are experienced. Severe hypoglycemia can occur from the mismatch of insulin timing with the postprandial glucose peak. Because of the delay in gastric emptying, pramlintide alters the postprandial glucose peak. If insulin reaches its peak before the peak in glucose concentration, postprandial hypoglycemia may occur, usually within 3 hours of pramlintide injection. Pramlintide is contraindicated in patients with gastroparesis. Treatment of type 2 diabetes includes management of dyslipidemia, hypertension, obesity, insulin resistance, and hypercoagulability as well as glycemic control.11 The mainstays of therapy are education, diet, exercise, and achievement and maintenance of a desirable body weight. These therapies have no side effects, although they are difficult to sustain over time. Hyperglycemia may be reversed with weight loss of as little at 4 kg.11 Early diagnosis and prompt initiation of treatment are associated with better sustained glycemic control and fewer long-term complications. Treatment of diabetes should be individualized to achieve an HbA1c level as close to the nondiabetic range as possible, and a value of 7% or greater should be a “call to action.”11 This target should be modified for patients with a significant risk for dangerous hypoglycemia or with a limited life expectancy. While recommendations for diet and exercise are being maximized, pharmacologic intervention should be considered. Consideration of the patient’s place along the continuum of the natural progression of type 2 diabetes and the mechanisms12 that are contributing to hyperglycemia, will guide the selection of medication(s). Individualization of medications should be determined on the basis of effectiveness, safety, tolerability, and cost. The reduction of risk for complications of diabetes has been shown to be related to the level of glycemic control over time rather than to the use of any single medication or combination.11 Some agents used to improve glycemic control also have beneficial or detrimental effects on related conditions (e.g., obesity, elevated lipids). The variety of diabetes medications to supplement lifestyle changes enables treatment individualization for improved glycemic control, targeting each patient along the continuum of type 2 diabetes. Agents with different mechanisms can be combined to achieve greater glucose reduction. Metformin (Glucophage), a biguanide, is commonly the first-line oral medication for type 2 diabetes. The mechanism of action is suppression of hepatic glucose production, which typically results in lower fasting glucose levels. A reduction of approximately 1.5% can be expected in the HbA1c level. Side effects are typically mild and include temporary nausea and diarrhea. Metformin does not cause hypoglycemia and may promote weight loss. There is the rare but serious risk of lactic acidosis. For this reason, metformin must be held before the intravenous administration of contrast material and temporarily discontinued (up to 48 hours) after radiologic studies involving intravenous contrast dye. In addition, metformin should be discontinued if the serum creatinine concentration exceeds 1.4 mg/dL in women or 1.5 mg/dL in men. This creatinine threshold has been debated as being too conservative and thus restricts the use of metformin for many patients who might benefit. Use of the estimated glomeruler filtration rate (eGFR) instead has been recommended by professional societies and has been proposed to the FDA.12 Sulfonylureas help reduce blood glucose by stimulating insulin secretion and can also reduce the HbA1c level by approximately 1.5%. The most common side effects are weight gain and hypoglycemia. Persistent hypoglycemia is more frequent in older adults. The second-generation medications in this class are glyburide (Micronase, DiaBeta, Glynase), glipizide (Glucotrol), and glimepiride (Amaryl). They are once- or twice-daily medications. Meglitinides (repaglinide [Prandin], nateglinide [Starlix]) are nonsulfonylurea insulin secretagogues. These are shorter-acting agents that must be taken with each meal and should be withheld if the patient is omitting a meal. The risk for weight gain exists, but these medications are less likely to cause hypoglycemia because of their shorter duration. α-Glucosidase inhibitors (acarbose [Precose], miglitol [Glyset]) act in the small intestine, delaying the digestion of polysaccharides. The inhibition of starch and the sucrose enzyme causes lower postprandial glucose levels. To be effective, the pill must be taken with the first bite of a meal that contains carbohydrate. Alone, these medications will not cause hypoglycemia; but if they are used in combination with another medication and hypoglycemia occurs, glucose (not sucrose) is necessary for treatment. Sucrose would be blocked by the action of these agents, so patients are advised to carry glucose tablets or gel. Contraindications include inflammatory bowel disease, colonic ulceration, obstructive bowel disease, gastroparesis, and creatinine levels above 2 mg/dL. α-Glucosidase inhibitors typically result in a 0.5% reduction in HbA1c. The thiazolidinediones (TZDs) pioglitazone (Actos) and rosiglitazone (Avandia) are peroxisome proliferator-activated receptor γ (PPAR-γ) modulators. These agents improve the sensitivity of liver, fat, and muscle to both endogenous and exogenous insulin. Ultimately, this works to decrease insulin resistance, thereby improving insulin sensitivity and decreasing insulin levels. In addition, the TZDs reduce hepatic glucose output. Improvement of both fasting and postprandial blood glucose levels occurs over time and without stimulating insulin secretion. Side effects include weight gain and edema; thus, congestive heart failure is a contraindication. TZDs should not be used as first-line therapy if the patient is glucose toxic because it takes 4 to 8 weeks for the full effect. Advantages include the delay of beta cell exhaustion, decrease in insulin resistance, no hypoglycemia (if used alone), and improvement of triglyceride and HDL levels. Some studies have revealed a slight increase in low-density lipoprotein (LDL) levels with rosiglitazone. A disadvantage of this class is a possible increase in liver function enzymes. However, the recommendation is to monitor liver function test (LFT) results and to stop the drug if serum transaminase exceeds 2.5 times the upper limit of normal. LFTs should then be monitored until the results return to normal. Caution is to be exercised when TZDs are used with insulin because they may cause more peripheral edema and, in cardiac patients, congestive heart failure. In July 2010, an FDA advisory panel reviewed evidence citing the increased risk of heart attack with rosiglitazone. The panel voted not to withdraw the drug but to continue to market it with additional restrictions and warnings. Subsequently, in response to the findings of the Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycemia in Diabetes (RECORD) trial, which showed no elevated risk of myocardial infarction (MI) or death in patients taking rosiglitazone compared with standard-of-care diabetes medications, the FDA lifted the restrictions.13 The FDA has been conducting a safety review of a reported association between long-term pioglitazone exposure and bladder cancer. A history of bladder cancer is a contraindication to pioglitazone use. The incretin mimetics glucagon-like peptide 1 (GLP-1) agonists are a class of noninsulin injectable medications indicated for treating type 2 diabetes that is inadequately controlled with healthy diet, regular exercise, and metformin. The GLP-1 peptide, produced in the small intestine, stimulates insulin secretion in the fed state. It acts to stimulate insulin secretion, to suppress glucagon secretion, and to slow gastric emptying. Gastrointestinal side effects are common and related to the effect on gastric motility. Weight loss is also common, but hypoglycemia is not. Exenatide (Byetta) is given as a twice-daily subcutaneous injection 0 to 60 minutes before breakfast and dinner, starting with 5 µg and titrating to 10 µg after a month if needed. Liraglutide (Victoza) is a once-daily subcutaneous injection, taken without respect to mealtime. There are three once-weekly GLP-1 receptor agonists: exenatide (Bydureon), dulaglutide (Trulicity), and albiglutide (Tanzeum). The GLP-1 receptor agonists have been shown to contribute to weight loss and a reduction in systolic blood pressure, in addition to a reduction in HbA1c. Combination therapy with insulin is an area of active investigation. A higher dose of liraglutide is now marketed as Saxenda and is indicated as a treatment for weight loss, as an adjunct to diet and exercise. Side effects at the higher dose include nausea and hypoglycemia. A related class of medications is the dipeptidyl peptidase 4 (DPP-4) inhibitors. They work to release insulin and to decrease glucagon levels by slowing the inactivation of incretin hormones. These medications help to regulate insulin by affecting both alpha and beta cells in response to elevated glucose levels. This process then helps decrease preprandial and postprandial glucose levels. The newest class of oral agents to treat type 2 diabetes is the sodium-glucose cotransporter-2 (SGLT2) inhibitors. This class includes canagliflozin (Invokana), empagliflozin (Jardiance), and dapagliflozin (Farxiga). These once-daily pills cause increased excretion of glucose in the urine and thereby reduce plasma glucose concentration and may contribute to weight loss. The side effects include an increased risk for urinary tract infections (UTIs), genital infections in females, and hypotension. If a single agent does not result in adequate glycemic control, a second agent may be added and then a third. Many different combinations have received FDA approval, and several two-medication tablets are available. The use of a combination tablet may reduce insurance copayments and may improve compliance because of the convenience. All medications have side effects, and many of the newer agents are expensive. An ADA position statement summarizes the current treatment recommendations.12 When a patient who is taking several diabetes medications in addition to medications for other conditions is not achieving the glycemic targets, insulin must be considered. Many patients find the switch from pills to insulin to be daunting. Thoughtful discussion of their concerns, reassurance, and a relaxed environment in which to practice injections can ease the transition. Insulin, given in adequate doses, can reduce any glucose level to the desired range. Individuals with type 2 diabetes may need large doses (1 unit/kg or more per day) to achieve desired glycemic control. An evening dose of intermediate-acting (NPH) or long-acting (glargine or detemir) insulin may be added to one or more oral medications. In some cases, other medications are discontinued and a split-mixed regimen of NPH and regular insulin or NPH and a rapid-acting analogue is recommended. Other patients will use basal/bolus insulin treatment alone. In addition to the insulin products used in type 1 diabetes, U-500 regular insulin may be used in type 2 diabetes. This is five times as concentrated as other insulins, allowing a patient with severe insulin resistance to inject a smaller volume. The use of U-500 insulin concentrate requires careful documentation and communication of the doses in actual units as well as in volume of insulin, to avoid overdosage or underdosage by a factor of five. Insulin may be used as first-line treatment of the patient with type 2 diabetes in the following situations: HbA1c level greater than 10% or glucose range above 250 mg/dL, severe illness with associated complications, gestational diabetes, and fragile older adults. However, the most common reason for use of insulin in type 2 diabetes management is failure to respond to the oral antihyperglycemic agents. Combination therapy allows a more individualized regimen aimed at optimal glycemic control. Despite all the medications, however, a healthy diet and regular exercise remain the cornerstones of management of diabetes.
Diabetes Mellitus
Definition and Epidemiology
Pathophysiology
Clinical Presentation
Physical Examination
Diagnostics
Fasting Plasma Glucose*
Random Plasma Glucose
Oral Glucose Tolerance Test* (75-g Glucose, 2-hr Plasma Glucose)
HbA1c*
Normal
<100 mg/dL
<140 mg/dL
<5.7%
Prediabetes
100-125 mg/dL
140-199 mg/dL
5.7%-6.4%
Diabetes
≥126 mg/dL
≥200 mg/dL with classic symptoms
≥200 mg/dL
≥6.5%

Differential Diagnosis
Management
HbA1c level
<7%*
Premeal blood glucose level
80-130 mg/dL
Peak postmeal blood glucose level
<180 mg/dL
Lifestyle Interventions
Pharmacotherapy
Insulin Therapy
Type 1 Diabetes.
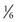 the TDD, but they can later be fine-tuned on the basis of postprandial blood glucose monitoring. The best approach to determination of mealtime insulin doses is counting the amount of carbohydrate to be consumed and calculating the dose on the basis of an individualized carbohydrate-to-insulin ratio.
the TDD, but they can later be fine-tuned on the basis of postprandial blood glucose monitoring. The best approach to determination of mealtime insulin doses is counting the amount of carbohydrate to be consumed and calculating the dose on the basis of an individualized carbohydrate-to-insulin ratio.
Type 2 Diabetes.
Medications for Type 2 Diabetes
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Diabetes Mellitus
Chapter 206
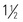 oz distilled spirits) per day for women and two for men; ingest with food to reduce the risk of hypoglycemia.
oz distilled spirits) per day for women and two for men; ingest with food to reduce the risk of hypoglycemia.



