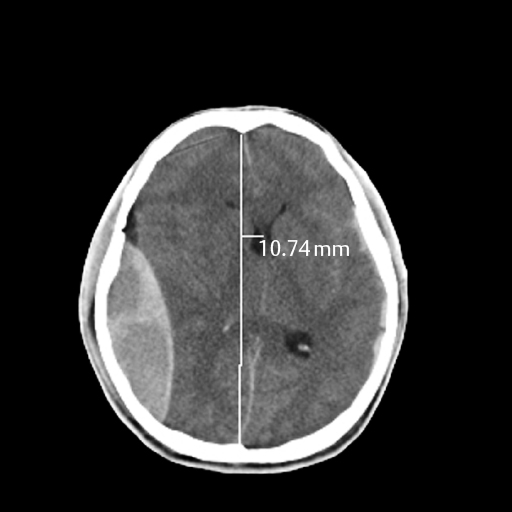
11 Lynn M. Serrano Intracranial hemorrhage has been a topic of significant research in the past several decades and includes multiple etiologies. Delayed hemorrhages previously discovered via angiography were considered relatively rare, yet with the advent of computed tomography (CT) scanning, the ease of diagnosis and follow-up has increased, as well as the incidence. Etiologies include both post-traumatic and nontraumatic. In this chapter we review the pathogenesis, diagnosis, management, and outcome of these lesions using three case examples. In 1891 Bollinger et al described delayed traumatic intracranial hemorrhages (DTICHs) as traumatische Spät-Apoplexie (“traumatic apoplectic event”) events using three criteria:1 Duret and other researchers expanded on Bollinger et al’s work by delineating the stages of DTICH.2,3 In the early 1900s, continued research led to theories of pathogenesis for DTICH, which we will discuss. Today, improved imaging has increased detection and diagnosis of DTICH, which was previously limited to operative and autopsy findings, and has enhanced our understanding of the disease. DTICH can be divided into epidural, subdural, and intracerebral hemorrhages. A delayed traumatic epidural hematoma (DTEH) exists when there is evidence of an epidural collection of blood on follow-up CT scan, not apparent on initial CT scan. This may occur with or without an underlying skull fracture. It has classically been described as “talk and deteriorate.” The symptoms vary according to the size and location of the hematoma and range from mild headache to focal neurologic deficits to deep comatose states. The delayed decline can occur anywhere from minutes to weeks after the initial traumatic event, most frequently between 6 and 48 hours after the trauma.3,4 Figure 11–1 Subdural hematoma. Figure 11–2 Epidural hematoma. The incidence of post-traumatic epidural hematoma in the pre-CT era occurred in less than 10% of all traumatic head injuries.5,6 The recent widespread use of CT scans reveals an incidence as high as 30%.7–9 DTEH specifically is estimated to occur in 2 to 10% of traumatic head injuries and as such has a predilection in males. It is more frequent in younger adults (average age: 27) relative to the elderly.10 One possible explanation for the accumulation is lifestyle differences; another is that, in advanced age, the dura mater can frequently adhere to the skull, preventing fluid accumulation.3 DTEH occurs most commonly in the temporal area (possibly secondary to thinner bone and the fragility of the middle meningeal artery) and with equal frequency in the frontal, parietal, and occipital areas.11,12 Delayed elevation of intracranial pressure, which may be caused by arterial bleeding or progressive edema, is a likely cause of the rapid deterioration these patients experience. Damaged vessels, initially in vasospasm, would allow increased blood flow and hematoma formation once the vasospasm has resolved.5,13–16 Hematomas also occur secondary to damage to superficial veins running along the arteries in the bony grooves, or damage to venous sinuses. Venous bleeding is slower and could account for the delayed detection of these bleeds. Patients initially in shock would be resuscitated, and efforts reversing the hypovolemic state would encourage bleeding from a damaged vessel. Measures taken to treat and relieve elevated intracranial pressure, including surgical and medical techniques, decompress the brain and create new space for subsequent bleeding as the tamponade effect is reversed.5,6,15 Piepmeier and Wagner17 showed that 10% of patients with an evacuated traumatic extra-axial hematoma developed a second surgical hematoma at an alternate site within 24 hours of the original surgery. Skull fractures could act as a decompressive device for acute epidural hematoma, allowing blood to seep into the subgaleal space and delaying intracranial symptoms, leading physicians to incorrectly believe the diagnosis to be delayed epidural hematoma. The gold standard is craniotomy for the removal of the hematoma. The patients described by Ashkenazi et al16 recovered completely after evacuation of the bleed with a craniotomy. They were discharged in excellent condition, and follow-up scans showed no reaccumulation. Smaller bleeds can be observed. Outcome is dependent on the initial presentation, the size and location of the bleed, and the aggressive treatment by the physician. Unsuccessful treatment of elevated intracranial pressure with an initial negative CT occurs in as many as 60% of cases of DTEH and is often used as an indication for follow-up scanning.6 Overall mortality approaches 12%, yet 91% of initially noncomatose patients and 35% of initially comatose patients taken to the operating room have a good recovery.10 Delayed subdural hematomas have been difficult to diagnose because of the frequently subacute and chronic presentation of this entity. A delayed traumatic subdural hematoma (DTSH) is an acute subdural hemorrhage, not apparent on initial imaging, appearing on follow-up CT scans within 30 days of a traumatic event. It usually presents as a decline in mental status, but significant neurologic deficit, though rarely seen, is a late finding of DTSH. The majority of cases are identified incidentally on follow-up scans or while being evaluated for new onset headaches and mental status changes. Of completely evacuated acute subdural hemorrhages, 0.5% will develop subsequent delayed reoccurrence.3 DTSH is most commonly associated with other intracranial hemorrhages, mass lesions, and brain edema. As with acute subdural hematoma, DTSH is frequently attributed to the tearing of bridging veins. The detection of subdural hemorrhages is often delayed because initial resuscitation efforts reverse low flow secondary to systemic hypotension or shock from other traumatic injuries. The tamponade effect caused by elevated intracranial pressure would delay frank bleeding. Generalized traumatic edema and other intracranial lesions (i.e., hemorrhagic or mass effect) would cause just this type of tamponade. The evacuation or reversal of the offending agent, via medication, procedures (e.g., ventriculostomy), or surgery would allow expansion of the subdural hemorrhage appearing as a DTSH. Vascular malformations may also be traumatically induced and have been identified as causes of DTSH.18 Treatment of DTSH depends on the size, location, overall mass effect, and neurologic status of the patient. General guidelines for DTSH are similar to acute subdural hematoma. Symptomatic lesions, lesions over 1 cm thick, and lesions with more than 0.5 cm midline shift should be evacuated. Depending on the age of the hematoma and the presence of membrane formation on CT scan, burr hole drainage or bedside drain placement drainage may be an acceptable alternative to a full craniotomy. The outcome for DTSH is highly variable and very case specific, as most studies incorporate patients with different etiologies, hematoma subtypes, symptoms, and presentations.19 As previously discussed, the ambiguity and multitude of classification systems surrounding a diagnosis of “delayed” nature, as well as the varied courses of cases and the wide range of possible intracerebral locations, make a clear definition of delayed traumatic intracerebral hematoma (DTIH) difficult. Studies are under way to investigate the practicality of using contrasted CT scans and/or magnetic resonance imaging (MRI) to detect future sites of DTIH. Lipper and Kishore’s suggested definition of DTIH20 mandates an initial CT scan with lesions smaller than 1 cm (including completely negative CT scans) and subsequent identification of a high-density intraparenchymal lesion on follow-up imaging. Fukamachi and Nagaseki21 divided traumatic intracerebral hemorrhages into four subtypes: The clinical presentation of patients with DTIH can be classified into four groups: The detection of DTIH has increased significantly with improved imaging techniques. The incidence is highly dependent on patient selection and project inclusion criteria, as well as the timing, availability, and quality of imaging. Delayed post-traumatic intracerebral hemorrhage is the fourth most common cause of all intracerebral hemorrhages after hypertension, vascular malformations, and alcoholism, in that order.13 As many as 50% of post-traumatic intraparenchymal enhancing lesions will develop into a hematoma.3 Since the advent of CT scanners, the overall incidence of delayed post-traumatic intracerebral hemorrhage is noted between 1.7 and 7.4% of all closed head injuries. It is generally accepted that ~10 to 20% of patients presenting with a GCS of less than 8 will continue to develop DTIH (group III and IV).22,23 There is little reliable data on the incidence of DTIH associated with group I and II patients. Although the severity and extent of trauma sustained are variable, head motion at the time of impact is significant for the development of DTIH.11,24 Bollinger et al initially hypothesized that necrotic brain softening around traumatized blood vessels led to DTIH.1 Microscopically damaged blood vessels, including traumatic aneurysms, may develop into DTIH. Post-traumatic dysregulation of cerebral blood flow leading to vasodilatation, caused by focal hypoxia and hypotension, and subsequent increased intravascular pressure due to resuscitative efforts, leads to elevated intracranial pressure and possible hemorrhage.25–27 Coagulopathy will cause poor hemostasis and clotting and can develop into a DTIH in 70 to 80% of patients.27,28 Locally damaged brain will release thromboplastic substances, which will produce intravascular coagulation. Such coagulation will lead to small areas of infarct, injuring the brain and restarting the cycle. The small infarction also creates additional space for possible expansion of the hematoma or future hemorrhagic conversion. Treatment is not significantly different from other traumatic intracranial hemorrhages. Intracranial pressure (ICP) monitoring is indicated for all groups, as rapid deterioration may be preventable with close monitoring and treatment of ICP. Any coagulopathies or other medical problems should be managed concurrently. Treatment is based on the clinical presentation, as follows:3 Prolonged low cerebral perfusion pressure directly correlates with poor outcome. Mortality rates as high as 75% are noted with group III and IV patients. Poor quality of life and vegetative states are not uncommon. The degree of secondary insult caused by the natural cascade of brain injury only adds to the underlying damage done by the hematoma itself and the associated mass effect. Coagulopathy needs to be corrected as soon as possible to decrease morbidity and mortality.27–29 Prompt intervention where appropriate is our only current tool to counteract the natural progression of DTIH. The clinical signs of nontraumatic intracranial hemorrhages usually occur spontaneously and are equally as unexpected as traumatic hemorrhages. Bleeding is usually short-lived and is tamponaded by anatomical and physiological means. Hemorrhages are best described, and treatment planned, based on their location and size, as identified on CT scans. It is estimated that 20% of patients with a previous intracranial hemorrhage will rebleed.30–32 Etiologies are widespread for spontaneous intracranial hemorrhages. However, delayed or recurrent nontraumatic hemorrhages are not as common and can be categorized as those that are due to anticoagulation treatments, subarachnoid hemorrhage, and other underlying medical conditions, including hypertension, infarction, tumors, migraines, or amyloidosis, and those that occur after invasive procedures. The general guidelines for evacuation of an ICH are
Delayed Intracerebral Hemorrhage
 Delayed Traumatic Intracranial Hemorrhage
Delayed Traumatic Intracranial Hemorrhage
 Delayed Traumatic Epidural Hematoma
Delayed Traumatic Epidural Hematoma
Pathogenesis
Treatment
Outcome
 Delayed Traumatic Subdural Hematoma
Delayed Traumatic Subdural Hematoma
Incidence
Pathogenesis
Treatment
Outcome
 Delayed Traumatic Intracerebral Hematoma
Delayed Traumatic Intracerebral Hematoma
Incidence
Pathogenesis
Treatment
Outcome
 Delayed Nontraumatic Intracranial Hemorrhages
Delayed Nontraumatic Intracranial Hemorrhages
< div class='tao-gold-member'>
Delayed Intracerebral Hemorrhage
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree