Fig. 5.1
Single-unit recordings under two different anesthetics . Each tracing shows 1 s of data. Tracings recorded from the same patient during a bilateral DBS STN procedure (a and b). Tracings recorded during a GPi DBS in two different patients (c and d). a and c were recorded using low-dose Dex (∼0.1 μg/kg/h); b and d were recorded using low-dose continuous infusions of propofol (<20 μg/kg/min). The recordings with propofol are much less robust than the ones during Dex infusions
After the DBS electrodes are inserted, sedation can be increased and the frame removed. Implantation of the pulse generator and internalization of electrodes can be performed either immediately or as second-stage surgery under GA. Patients should receive their usual antiparkinsonian medication as soon as possible after the procedure to avoid possible deterioration in neurologic function and respiratory muscle impairment.
There is limited information on the incidence of intraoperative anesthetic complications during these procedures. A review of intraoperative anesthetic-related complications in a series of 158 cases of deep brain ablation or stimulation under sedation with propofol or Dex [20] found that intraoperative events occurred in 6.96 % of cases. These events included coughing, sneezing, aspiration, pulmonary edema, combative behavior and agitation/confusion, bronchospasm, angina, and intracranial hemorrhage. All of these have the potential of moving the electrodes and cannula in the brain and causing an intraparenchymal hemorrhage. Yet, with an anesthesiologist who understands the specific movement disorder of the patient, the surgical procedure, the need for quality, and who pays constant attention to the alertness of the patient, these effects can be minimized. In our experience, the only case of a complication was when the anesthesiologist was concentrating on the infusion numbers and not the patient.
The MER Procedure
Due to accuracy problems with direct CT or MRI targeting, visualization is generally only a first step in locating the target, whereas MER gives a much more detailed spatial and functional map. Several excellent descriptions of the MER technique exist [21–32]. Understanding the type of neuronal activity from regions immediately adjacent to the intended target is critical to the success of the procedure. Following are two case examples (one fairly typical and the other unusual and complex) illustrating the integration of diagnosis, technique, and anesthetic management. During a MER recording session, not only are recordings acquired from the target location but also from structures located above and below that location. These recordings from other structures are useful in helping to determine the correct sagittal, coronal, and axial positions of the target. At the time of the writing of this chapter, both patients are significantly improved and are still benefiting from the procedure. In addition to looking for spontaneous single cell firings, it is important to look for cells that respond to specific types of evoked activity given that the basal ganglia is composed of segments that are nonsensory motor. Finding cells that respond to voluntary patient movement and cells that respond to limb joint position (kinesthetic) are key in making sure the electrode will be placed in the sensory motor region of the specific target. Both voluntary and kinesthetic cells will either increase or decrease their firing rate during the activity and are only found in the sensory motor region of the specific nuclei. Voluntary testing is performed by asking the patient to move a particular body part while looking for changes in the firing pattern of the single unit under study. Kinesthetic testing is performed by moving an isolated joint and looking for changes in the single unit’s firing rate.
Target Structures for the Case Examples
For movement disorders surgery, there are three common targets : (1) the ventral intermediate nucleus (VIM ) of the thalamus, (2) the internal globus pallidum (GPi) , and (3) the STN. Each of these targets is thought to best treat a specific symptom of a movement disorder of a specific disease, although research is still underway to prove these hypotheses. Two of the three cases that will be presented in this chapter involve placing DBS electrodes in the GPi and, for the other case, in the STN. These cases were chosen because they demonstrate the extremes of movement disorder surgery. VIM is the primary target for tremor-related diseases such as essential tremor and one can still use the lessons from the cases below in a VIM case because it is the patient that dictates the anesthetic intervention and not the disease. To get a better feel for the procedures, we have included a short description of what type of physiology is encountered to show what can be lost if the anesthetic technique is not appropriately applied.
Although the complete GPi may be visualized on an MRI, the functional target location in the GPi is in the posterior and ventral region of the nucleus [33–39]. As the microelectrode is lowered into the brain along its recording trajectory toward the target location, the firing patterns from three anatomical structures must be recognized to confirm optimal placement: the striatum, the external globus pallidum (GPe), and the internal GPi. Figure 5.2 shows the anatomy of the GPi and the surrounding structures. The recordings on the right show representative firing patterns from each area. These are the critical physiologic markers in differentiating the structures. During placement of the DBS electrode, proximity to certain structures must be avoided such as the optic tract and internal capsule, which can render the therapy useless.
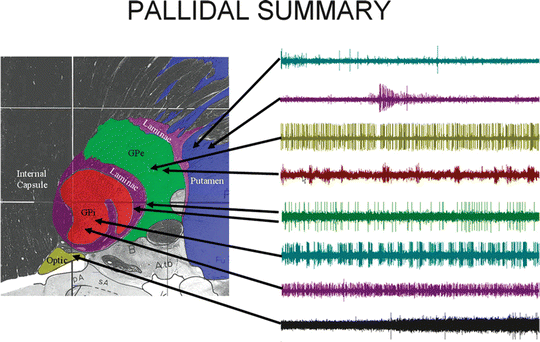

Fig. 5.2
A sagittal image , ∼21.5 mm from midline, through the GPi and associated anatomy. Traces to the right are representative firing patterns from each area. Each trace represents single-unit activity as recorded from a representative cell in that structure [68]
After the microelectrode mapping is performed, micro- or macroelectrode stimulation is performed prior to permanent DBS placement to ensure that the electrode is at a safe distance from the internal capsule and the optic tract. If the patient is awake and alert, they can easily respond by indicating when they see flashing lights or experience muscle contractions. If the patient cannot respond, they cannot indicate when the optic tract is being activated or if they are having muscle tightness. In these cases, EMG recordings are used to indicate if muscles are being activated by either direct stimulation of the motor fibers in the internal capsule, which is an adverse effect, or the contractions are not a direct result of the stimulation but an indirect effect of stimulation at the appropriate location in the GPi. Thus, it is critical that no muscle relaxant be used.
The functional target in the STN is in the middle of the structure between 10.5 and 13.0 mm lateral from midline [28, 32, 40–42]. Once again, as the microelectrode is slowly lowered toward its target location, the neuronal firing patterns from three anatomical structures must be recognized to confirm optimal placement: the thalamus, the STN , and the substantia nigra pars reticulata (SNr). Figure 5.3 shows the anatomy of the STN and the surrounding structures. The waves on the right show representative firing patterns from each area, which are the critical physiologic markers in differentiating the structures. If the DBS electrode is placed too medial and posterior within the STN, it can activate the sensory thalamus and/or medial lemniscus; if it is placed too lateral and anterior, it can adversely affect the internal capsule and render the therapy useless.
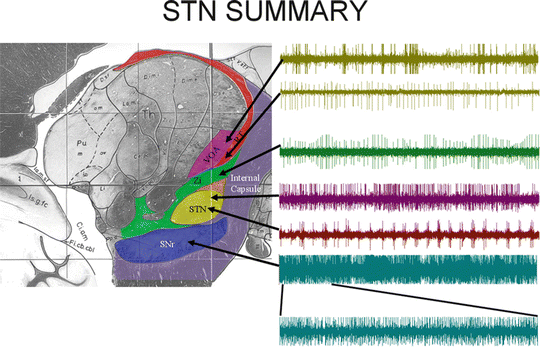

Fig. 5.3
A sagittal image, ∼12.5 mm from midline , through the STN and associated anatomy. Traces to the right are representative firing patterns from each area. Each trace represents single-unit activity as recorded from a representative cell in that structure. For the SNr, the recording is from multiple units [68]
Cases and Disorders
Case 1: Noncomplex (PD-STN): A 60-year-old male with a 10-year history of PD, well controlled on Sinemet until age 59.
Clinical symptoms of PD began on the patient’s right side starting with tremors in the upper extremity and rigidity in both the upper and lower extremities. As the disease progressed, symptoms became as problematic on the left side and started to affect the patient’s ability to ambulate. One year prior to surgery, levodopa-induced dyskinesias began in the head and face, followed by a progressive difficulty swallowing. At the time of the surgery, the patient was on Stalevo (a mixture of carbidopa, levodopa, and entacapone) 37/5/150/200 q.i.d., Requip 4 mg t.i.d., Amantadine 100 mg t.i.d., and Neurontin,300 mg t.i.d. Due to the medication-induced symptom of dyskinesia and the minimal amount of benefit, surgical implantation of a DBS electrode in the STN was then planned.
Parkinson’s Disease
PD is a slowly progressive degenerative disorder of the BG. Nerve cells in SNc produce dopamine, which is transported to the input of the BG (striatum). In PD, for reasons not yet understood, the dopamine-producing nerve cells of the substantia nigra die off. The clinical signs of tremor, bradykinesia, and rigidity do not fully become apparent until significant dopaminergic neuronal cells are lost [43–45]. Medications are the first line of treatment to alleviate symptoms of PD, yet in many patients who have been responding to medications, their symptoms usually begin to gradually worsen with time. As they become more pronounced, patients may start to have difficulty walking, talking, or completing other simple tasks. Surgery should be considered when the patient develops moderate to severe motor fluctuation, medication-induced dyskinesia, medication refractory tremor, or intolerance to medication. Levodopa-sensitive symptoms may be more likely to respond to surgery [46], although in our experience, surgery in the STN and the GPi for PD has demonstrated a benefit to dopa-induced symptoms [41, 42]. Continued refinement of the knowledge of BG circuitry and PD pathophysiology has narrowed the focus of movement disorder surgery to three nuclei: (1) the thalamus, (2) GPi, and (3) the STN. The STN is the preferred surgical target for DBS electrode placement in PD [41, 47–50]. Serious complications such as hemorrhagic bleeding associated with STN-DBS electrode placement is relatively uncommon [51]. Hypertension must be treated prior to surgery because of the risk of hemorrhage [52, 53]. PD patients commonly suffer from orthostatic hypotension, contributed to by the use of levodopa and dopamine agonists, as well as other autonomic disturbances [54–56]. Respiratory dysfunction is well known in PD [55]. This includes an obstructive ventilation pattern, dysfunction of upper airway musculature, rigidity, bradykinesia , and dystonia of respiratory muscles [57]. These problems are exacerbated by withdrawal from antiparkinsonian medications.
Procedure and Decisions
The patient arrived at the hospital on the morning of surgery “off” of all PD medications from 7:00 p.m. of the night before. During frame placement, propofol was given in 20 mg boluses for sedation and monitored by the anesthesiologist. A foley catheter was also placed while the patient was sedated. The patient was then taken to the CT scanner (an MRI was performed at an earlier visit) with propofol given as needed to keep the patient relaxed. After the CT, the patient was brought to the OR, transferred to the bed and positioned with the frame also locked to the bed. It is important that the patient feel comfortable with both their ability to breath as well as with the position of their neck and back because they will be locked in that position for the duration of the surgical procedure, which can be several hours. When the patient is comfortable, either a propofol infusion or bolus doses are given until the dura is opened. Once the dura is incised (about 10–15 min before the MER is to start), the propofol is stopped to allow the patient to be awake for testing. Recording tracts in this patient included one on the left side and three on the right side. This difference in the number of recording tracts can be caused by potential asymmetries in anatomy, effects of nonlinear errors in imaging, or brain shift during the procedure. It is hard to pinpoint the exact reason, but in about 15 % of our cases we find this discrepancy. Each move requires the surgeon to remove the recording system and electrode from the head and place a new tract in the brain. Any time an electrode or cannula is placed in the brain, the chance for hemorrhage increases. Thus, it is critical to keep the BP below 150 mmHg systolic. On the left side, 4.9 mm of STN were encountered with four kinesthetic cells. On the right side, the first tract had 4.6 mm of questionable STN and no kinesthetic cells. The second tract had 5.7 mm of STN with three kinesthetic cells, and the third tract had 1.3 mm of STN with no kinesthetic cells. At the start of the second tract on the right side, the systolic BP increased above 150–165 mmHg. Recordings were halted and 10 mg of labetolol were given until the systolic BP dropped to 135 mmHg. Subsequent stimulation and recording required no changes or additions to the anesthesia. All of our PD patients have nasal cannula O2 administered throughout the procedure and SpO2 monitoring.
The DBS electrode was placed in the first recording tract on the left side and the second recording tract on the right side. These placements were chosen due to the number of kinesthetic cells and length of STN encountered. Once the DBS electrodes were placed, they were tested with an externalized stimulator (Medtronic Dual 7240 stimulator, Minneapolis, MN). Testing is performed in a sequential bipolar fashion (−0,+1: −1,+2; −2,+3) using a pulse width of 60 μs and a frequency of 180 Hz. Voltage is slowly raised to 4 V. For this patient, no continuous adverse effects were noted with stimulation up to 4 V. There were some transient sensory paresthesias in the arm that lasted for about 5–15 s. Transient adverse effects are acceptable since the device is never supposed to be turned off. We were able to get improvements in bradykinesia and rigidity at the −1,+2 for the left side and both −0,+1 and −1,+2 on the right side. Postoperatively the patient was improved by 72 % based on the unified Parkinson’s disease rating scale part III (a common motor classification system for PD patients).
Case 2: Complex: A 14-year-old boy with methylmalonic acidemia (MMA) was diagnosed at age 3 months. His condition was well controlled and in good health until acquiring H1N1, when he subsequently developed pancreatitis, sepsis, and in turn bilateral BG strokes.
As a consequence of the strokes, he developed spastic quadriparesis for which a baclofen pump (a common treatment for spasticity) was placed without benefit. He was admitted for worsening dystonia, and resistance to multiple medical therapies. Due to the severity and worsening dystonia, including fixed posturing and dynamic spasms, it was decided to move forward with bilateral GPi stimulation. During the time between the baclofen trial and the decision to move forward with DBS electrode implantation, the patient’s respiratory status deteriorated somewhat as well.
Dystonia
Dystonia refers to a syndrome of involuntary sustained or spasmodic muscle contractions involving cocontraction of the agonist and the antagonist muscles [58–60]. The movements are usually slow and sustained, and they often occur in a repetitive and patterned manner. However, they can be unpredictable and fluctuate. The frequent abnormal posturing and twisting can be painful and functionally disabling. Regardless of the causes, the dystonic contractions can have a chronic course and can lead to severe persistent pain and disability. Because each type of dystonia is treated in a different manner, the distinction between the various types is therapeutically important [61–66]. On the basis of its clinical distribution, dystonia is classified as focal dystonia, segmental dystonia, multifocal dystonia, generalized dystonia, and hemidystonia.
Systemic medications benefit about one-third of patients and consist of a wide variety of options, including cholinergics, benzodiazepines, antiparkinsonism drugs, anticonvulsants, baclofen pump, carbamazepine, and lithium [67]. Many patients with dystonia realize an inadequate response to those treatments [68]. For such patients whose symptoms are sufficiently troublesome, surgical treatment can be used to reduce symptoms and improve function. For dystonia, stimulation is primarily directed at the GPi, which has been the most thoroughly studied stimulation site to date.
Procedure and Decisions
This particular case was one of the most complex of all that we have experienced for DBS electrode implantation. The patient was extremely tenuous metabolically due to the MMA and required anesthesia for the procedure due to excessive movement. The anesthetics themselves can cause not only poor recordings but also adverse metabolic effects. In this case, the surgeon, neurophysiologist, anesthesiologist, critical care physicians, neurologists, and social workers all met to plan the pre-, intra-, and postsurgical management of the patient. Each group discussed their particular needs for the case and the effects each would have on the patient. As discussed previously, the most common issue facing the anesthesiologist in most DBS cases is BP control. A plan was made to use Dex initially and if that proved unacceptable, then propofol would be used if the patient could tolerate it. Due to the underlying metabolic issues, it was questioned whether a controlled amount of Dex could be given to allow for patient comfort and also the ability to record. Also, one of the main problems with Dex is the potential for causing hypotension in a patient. This is not a major issue in PD patients, where this effect is usually helpful, but in children, it needs to be a consideration. Three days prior to the procedure, the plan was to try and wean the patient off of some medications, including high doses of benzodiazepines, which proved unsuccessful.
On the morning of surgery the patient was taken directly to the CT scanner and intubated. Anesthesia included 3 mg of midazolam, 10 mg of etomidate initially followed by another 5 mg, 12 mg of cisatracurium, and 100 μg of fentanyl. The patient was maintained on sevoflurane (1.5 %) for the placement of the frame, and also while they were in the CT area. Prior to moving the patient to the CT area, Dex was started at 0.7 μg/kg/h with remifentanil at 0.1 μg/kg/min. Five minutes after this the sevoflurane was stopped. This infusion continued up to the creation of the first burr hole in the operating room. At this point the Dex was reduced to 0.1 μg/kg/h and the remifentanil was stopped. The purpose of reducing the Dex at this time was to allow about 10–15 min to slightly awaken the patient for the recordings. Once the burr hole was created and the dura incised, the BP was confirmed to be 127/77 and the initial cannuli were inserted into the brain, followed by the microelectrode. At this point (with the patient still intubated yet alert and able to follow simple commands) the patient exhibited no spasms and even had his eyes open. Once the electrode entered the brain, “burster” cells were recorded indicating: (a) that the tip was in the GPe, and (b) that the sedation level (Dex at 0.1 μg/kg/h) permitted the ability to record single units while still preventing excessive movements. Recordings continued, moving through GPe, through the laminae separating the two structures and into GPi. Because the firing rates of the GPe and the GPi are similar, the ability to record kinesthetic cells is critical to distinguish between the two. Keeping all anesthetics constant, kinesthetic testing was done on all cells encountered. Eight distinct single units were located on the first side with kinesthetic activity noted on three of them. Recording was stopped near the base of the GPi due to an increase in BP to 168/120 mmHg, which was most likely due to a continued wearing off of the Dex and the patient becoming more alert to his surroundings. Advancing of the electrode stopped and two 6-mg doses of hydralazine and a 1.5-μg/kg/min infusion of sodium nitroprusside was needed to bring the pressure down. The pressure eventually stabilized to 114/50 mmHg. The Dex was increased to 1 μg/kg/h and the remifentanil was increased to 0.1 μg/kg/min. Upon exiting GPi, a border cell was noted and then the optic tract 2 mm below. Because it was impossible for the patient to describe muscle activity to the team during macrostimulation (stimulation testing through the permanent DBS lead after it is placed to assure there are no significant adverse events), we used EMG recordings to assess muscle activation. No direct driving activity was noted at 5 Hz, while a minor thumb contraction was noted at 130 Hz and 7.0 V. 5 Hz is used because it is the lowest output of the test stimulator and will give muscle contractions that follow the stimulus train. If this occurs, the muscle activity is related to direct activation of the internal capsule and demonstrates that the electrode is too medial and posterior. If no muscle activity is noted, then the contracture noted previously is most likely due to stimulation of the GPi. In our experience, this has been shown to be a positive indicator of electrode placement, as is a contraction of the nasal labial fold, when stimulation is above 5.0 V. With this information the electrode was then placed. The Dex was then increased to 1.4 μg/kg/h and the remifentanil was adjusted to 0.07 μg/kg/min for the closure of the first burr hole and the creation of the second burr hole. Once the second burr hole was created, the Dex was reduced to 0.7 μg/kg/h and the remifentanil was stopped. Nitroprusside was continued at 0.5 μg/kg/min. No further changes in anesthetic were needed during the recording or stimulation on this side. The patient was able to open his eyes during the procedure. On this second side, 12 distinct GPi cells were recorded, and a small area of the internal laminae that separates the external and internal segments of the internal GPi was detected. Kinesthetic activity was noted on five of those 12 cells. The optic tract was also noted about 1.7 mm from the base of the GPi. Stimulation testing, similar to the first side, was performed with no adverse events. At this point, sevoflurane was added at 0.7 % for the remainder of the procedure, which included the implantation and tunneling of the connecting wires and IPG.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








