Past
Current
Future
Surgery
Aggressive definitive surgery
Damage control surgery
Prehospital control of bleeding
Improvement of haemostatic agents.
Improvement of interventional radiology with hybrid theatre
Haemostatic agents
Coagulopathy
“Catch up” correction in coagulopathic patients
Massive transfusion protocols
Genetic guidance to treatment.
TEG return
Early treatment (prehospital)
Perfusion/ BP
Aggressive resuscitation with clear fluids
Early blood and blood products
Development of the optimal oxygen carrier
Restriction of clear fluids
Inflammation
None
Decrease fluids that stimulate an immune response
Targeting genetic targets
Early treatment
Coagulopathy in polytrauma is complex and has multiple components. It is divided into dilutional coagulopathy and non-dilutional coagulopathy. Dilutional coagulopathy is due to diluting the clotting factors with crystalloids, colloids, and blood administration and is directly related to the amount of fluids that was received. To avoid dilutional coagulopathy during a massive transfusion, fresh frozen plasma and platelets are transfused as part of a transfusion protocol. Non-dilutional coagulopathy is the initial type of coagulopathy that develop in the poly trauma patient due to the systemic factors and hypoperfusion. This lead to acidosis and the activation of protein C. Activation of protein C causes coagulopathy by inhibiting plasminogen activators and Factors V and VIII.
“Truncated laparotomy” was first described by Stone and colleagues in 1983 for patients with clinical coagulopathy. Rotondo and Schwab used the term “damage control laparotomy” in 1992 mainly referring to an abbreviated procedure for patients with massive hemorrhage [9]. It created the concept of a limited initial operation to stop the hemorrhage and control the contamination rather than definitive repair. The aim is not only to take the patient alive out of the operating theatre but also to limit the possibility of postoperative multiorgan failure. Initially Rotondo et al. described damage control for abdominal trauma in three stages (initial laparotomy, resuscitation in ICU, and definitive surgery) [9, 10]. Johnson and Swab later on added a fourth phase which include the pre-theatre phase. Currently it has been expanded into five stages: Stage 1: Patient selection and resuscitation in Emergency Department; Stage 2: Initial operation; Stage 3: Resuscitation in ICU; Stage 4: Definitive surgery; and Stage 5: Abdominal wall reconstruction. Currently the principles of damage control applies not only to abdominal but also to thoracic, vascular, orthopedic, and neurosurgical trauma.
Patient Selection
Early and appropriate identification of patients who can potentially benefit from damage control is critical and should be made early, best if it is done in the emergency department. There is no isolated “physiological threshold” that should trigger damage control, but a combination of injuries, physiological parameters, expected hemorrhage and response to treatment, as well as the surgeon’s clinical decision should be the key factors. Patients with indications for damage control surgery that would undergo definitive surgery inevitably would have a poor outcome or lead to unplanned damage control. On the other hand, overzealous use of damage control deny patients a single definitive procedure, increase ICU and hospital stay and cost, increase morbidity (intra-abdominal infections, fistulas, hernias) and mortality. Our current emergency department indications for damage control are: injury severity score >25; persistent hypotension with systolic blood pressure of less than 70 mmHg; temperature of <34 °C; pH < 7.2; lactate of >5 and worsening; INR and PTT >50 % of normal. On occasion, damage control is applied as part of disaster management aiming to free up overwhelmed resources. Patients that were not initially triaged for damage control in the emergency department might undergo damage control in theatre due to the following: Worsening of physiology (decreased temperature, decreased pH, increased lactate); Increasing inotropic requirements; continuous of massive transfusion protocol (ten or more packed red blood cells in less than 24 h or replace half of the blood volume in less than 3 h); inability to control hemorrhage that force packing; and operative time greater than 90 min. Overall it is estimate that 10 % of major trauma patients might benefit from damage control surgery.
Damage control resuscitation includes ATLS resuscitation with the ABCs. In military resuscitation where a large source of bleeding is visible they adopted the <C > ABC resuscitation approach with immediate control of the hemorrhage. Damage control resuscitation classically consists of permissive hypotension, hemostatic resuscitation, and damage control surgery for control of bleeding and contamination.
Recently two other components have been added to damage control resuscitation, namely rewarming and correction of acidosis. The goal is to minimize the physiological insult on the patient and to improve outcome by decreasing the volume of blood loss. If all three components are adhered to, the amount of blood and blood products used will be reduced, with an improvement in 24 h mortality and a 2.5-fold improvement in 30-day mortality [11].
Permissive Hypotension
The aim of permissive hypotension is to maintain a lower systolic BP than normal until surgical control of the hemorrhage can be obtained. It was initially described in 1918 by Cannon et al. [12] and later reinforced by Bickell et al. in 1994, who showed an 8 % absolute reduction in mortality in hypotensive patients with penetrating torso trauma and delayed resuscitation until surgical control of the hemorrhage was obtained [13]. With hypotensive resuscitation only a limited amount of fluids are given to the patient. Aggressive resuscitation with saline-based fluids is associated with increased blood loss due to coagulopathy and the displacement of established clots. It is also associated with acidosis, cardiac dysfunction, increased abdominal compartment syndrome, severe inflammation and ARDS, multiple-organ failure, and increased mortality [14]. On the other hand restricting fluids contributes to systemic ischemia due to hypoperfusion. This ischemic stress can also cause coagulopathy and a systemic inflammatory response. An attempt to bridge this hypoperfusion with the use of vasopressors worsens outcome. The theory is that it causes capillary vasoconstriction, which worsens cellular ischemia [15, 16]. Some studies suggest that permissive hypotension should only be practiced for 90–120 min to avoid organ damage. The role for permissive hypotension is well described in penetrating trauma but the evidence is not clear on blunt trauma. It is yet to be established what would be safe in patients with head injuries and known cardiac patients. Morrison et all looked at 90 patients where the intraoperative mean arterial pressures were kept at 50 instead of 65 and found that less clear fluid and blood products were administered, and improved early postoperative survival and a trend to improved 30 day survival [17]. The ideal fluid for resuscitation should be a fluid that has oxygen-carrying capabilities, is able to reduce tissue ischemia and will not cause coagulopathy or inflammation. Unfortunately despite many pharmaceutical trails there is no perfect fluid at this stage. The overall current consensus among trauma surgeons is that crystalloids and colloids must be limited as much as possible. In the prehospital environment according the EAST and other guidelines, IV access should only be obtained in transit to hospital if the patient is not entrapped, and radial pulses should be used as indication for 250 ml fluid boluses. However, the controversy with crystalloids vs. colloids is an ongoing debate. Some studies suggest that colloids are better than crystalloids [18]; other studies suggest that colloids—especially the starches—cause coagulopathy and acute renal failure. A meta-analysis done to look at all fluids used for resuscitation in patients in hemorrhagic shock found there was no fluid that was better than another in mortality endpoints [19]. Initial studies suggested hypertonic saline to be the ideal resuscitation fluid in patients with traumatic brain injury and hypotension, but studies that were conducted by the Resuscitation Outcomes Consortium (ROC) were stopped due to safety concerns. Currently hypertonic saline is not recommended for hypotensive resuscitation [20, 21].
Hemorrhagic Resuscitation
Early resuscitation with blood and blood products has been shown to reduce coagulopathy and mortality. Whole blood makes up the best resuscitation fluid and is being used by the US military. Due to limited availability and short shelf life of whole blood it is not readily used and components of blood are transfused separately. Military studies have shown a 46 % reduction in mortality in patients that required massive transfusion and were resuscitated with FFP–Blood in a 1:1 ratio. Platelets are added on to the ratio FFP–Blood–Platelets at 1:1:1 although there is very limited evidence to support this practice at the time of this writings. Part of the controversy is that the ratio does not constitute whole blood and only makes up a half to two-thirds of the active elements in blood. Currently, the first prospective study looking at the optimal ratio of blood, FFP, platelets, and other products is PROPPR (Pragmatic, randomized optimal platelet and plasma ratios) study. The thrombo-elastogram should still be used to guide component therapy [22]. Massive blood transfusion is defined as ten or more units of red blood cells in 24 h or more than 50 % of the blood volume in 3 h. Early activation of the massive transfusion protocol is essential in patients with ongoing bleeding; usually after the second unit is administered, the practitioner should activate the protocol if it is anticipated that the patient will need a massive transfusion. Early activation decreases the amount of blood products required as coagulopathy is better controlled due to early component transfusion. The recognition of the impact of coagulopathy in trauma developed an interest in pharmaceutical agents that could help with clotting:
Factor VII a: Factor VII a plays a major role in the initiation phase of clot formation. Several studies confirmed it decreased transfusion requirements in blunt trauma but not in penetrating trauma. However, it has shown no effect on mortality [23, 24] and is very expensive.
Fibrinogen: Fibrinogen is depleted earlier than other clotting factors and can be given as fibrinogen concentrate or as part of cryoprecipitate (Fibrinogen, factor VIII, and Factor XIII and von Willebrand factor). Current evidence shows no benefit unless thromboelastography has shown a deficiency, and the large number of required donors increases the risk for infections.
Tranexamic Acid: Tranexamic acid inhibits plasmin, which plays a vital role in coagulation and inflammation. A large randomized control study (Crash-2) showed a reduction in mortality when administered early in bleeding patients [25]; however, further high powered studies are required to confirm its role. Similar antifibrinolytics include aprotinin and aminocaproic.
Calcium: Calcium plays a vital role in coagulation and is an important cofactor for various enzymes. Levels must be continuously monitored during massive transfusion and should be replaced when depleted. Component resuscitation in a coagulopathic patient should only be done using thromboelastogram or ROTEG.
Early control of hemorrhage is also part of hemorrhagic resuscitation. Tourniquet has recently been extensively used to control extremity bleeding. Local hemostatic agents have been developed and form a large part of hemorrhage control.
It is a very commercially driven part and there are numerous products that can be used and include amongst others:
Factor concentrators [26]: These products concentrate the clotting factors by rapidly absorbing the water in the blood.
Mucoadhesives: These products adhere to the adjacent tissues to form a plug to bleeding wounds.
Pro-coagulant supplementors: These products contain clotting factors that are delivered into a bleeding wound and promote clotting. Local hemostatic agents, while they do not replace surgical techniques and should not be used with active arterial bleeds as the sole treatment method, can be helpful. The development of cellulose minisponges that can be placed into cavities with severe incompressible hemorrhage have proven to have a significant reduction of hemorrhage and have reduced transfusion requirements and improved outcome [23]. High-frequency argon beams can be also used on raw surfaces.
The Development of Hemorrhagic Shock Management
Rewarming the Patient
Preventing or reversing hypothermia is part of resuscitation. Rewarming the patient should be started at the torso rather than the extremities to prevent acidosis and hypotension due to peripheral vasodilatation. The following three methods of rewarming are most frequently used:
1.
Passive external warming (blankets and ambient temperature)
2.
Active external warming (warm air devices)
3.
Active internal warming (warm fluids and blood, irrigation via an intercostal drain, warm fluid gastric lavage, or washing of the abdomen with warm fluids)
Correcting Acidosis
The best way to correct acidosis is stopping the bleeding and ensuring optimal resuscitation and restoration of end-organ perfusion. Currently, base excess and lactate are used most commonly to monitor end points of resuscitation. The initial levels and the level of clearance of lactate correlate with the mortality of the patient. There is no convincing data to support the use of bicarbonate in patients with a pH less than 7.2.
Damage Control Surgery as a Team Approach
Damage control surgery is a team approach where the main goal is to limit surgery time, to control the bleeding and contamination and minimize the physiological insult.
Each team member plays a vital role in ensuring the best possible outcome. The following steps must be adhered to:
1.
The operating theatre is warmer than usual.
2.
The nursing staff must ensure that all possible equipment that may be required is readily available (this includes, but is not limited to gastrointestinal staplers, nasogastric tubes for shunts, suture materials, vacuum dressings, vascular sets, etc.). Patients with torso injuries must be prepped and draped from the chin to the knees (Fig. 14.1), and areas not being operated on must be covered. The nursing staff must ensure that accurate records are kept of swab count and all blood must be removed for cell saving purposes. Folded swabs must be kept on the patient upon opening the peritoneal cavity, warm blankets and fluid must be available to prevent hypothermia, and local hemostatic agents must be available as required.

Fig. 14.1
Draping of the patient should allow access to all potential cavities. Areas not used should be covered to prevent hypothermia
3.
The anesthesiologist must continue resuscitation using the damage control principles, prepare the autologous blood recovery system for cell saving (Fig. 14.2), ensure the surgeon is completely ready prior to administering any muscle relaxants or putting the patient to sleep, and give prophylactic antibiotics and repeat if the patient loses more than his blood volume. Perhaps the anesthesiologist’s team most important role is that of keeping the surgeon informed of the physiologic status of the patient (i.e., inotropic requirements, blood gases, and vital signs) as the surgeon may need to change to damage control if the surgical findings and response to treatment do not correlate. Is the surgeon missing something or is in the wrong cavity?
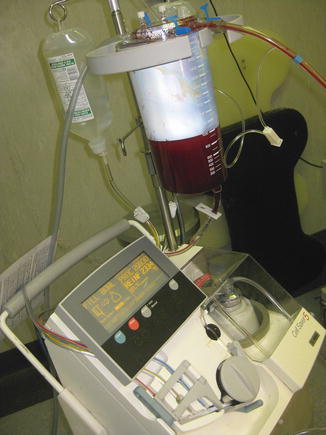

Fig. 14.2
Cell saver (Cell Saver® 5, Harmonetics, Braintree, MA)
4.




The surgeon must keep everyone in theatre informed about the injuries found and the future management plan, as well as have the ability to change to damage control if the injuries are more severe than initially anticipated or if the patient deteriorated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








